Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Compound A gives the following IR spectrum. Upon reaction with sodium borohydride, followed by acid workup, the product B with the 'H NMR
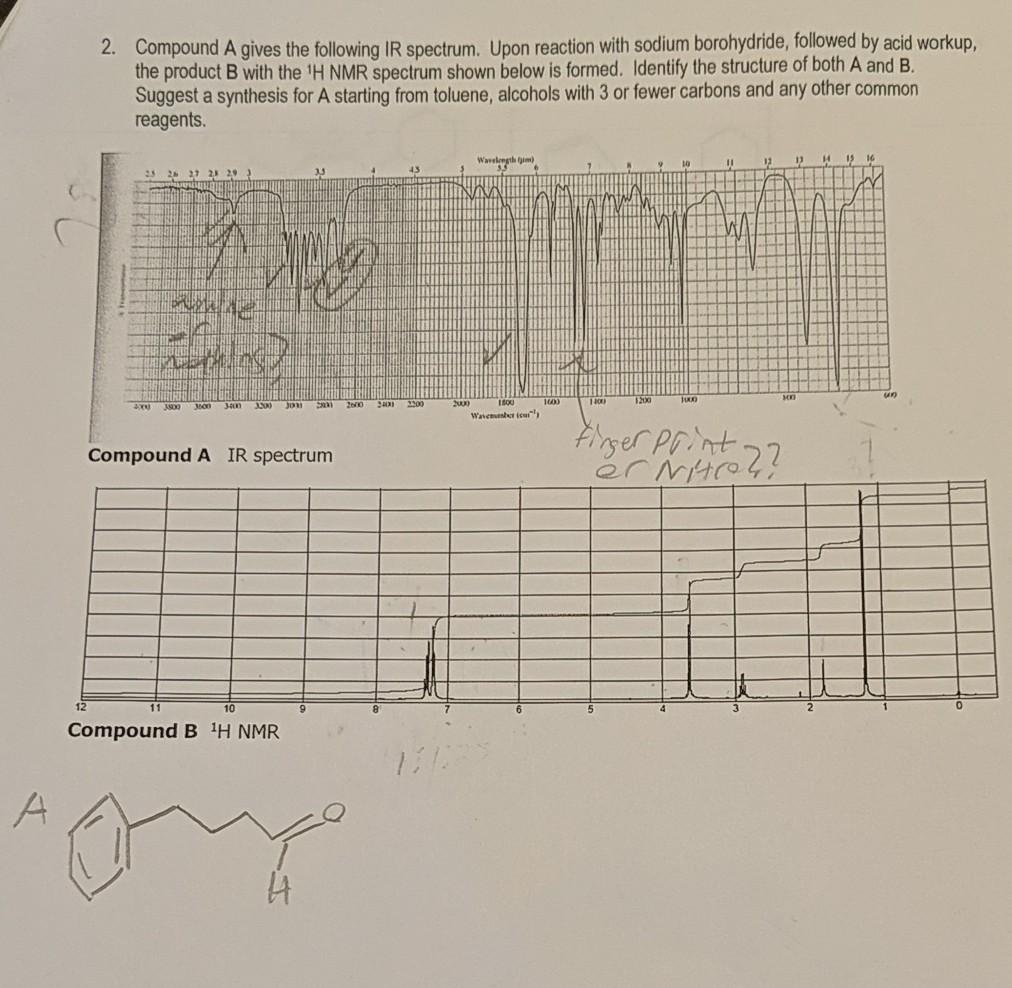
2. Compound A gives the following IR spectrum. Upon reaction with sodium borohydride, followed by acid workup, the product B with the 'H NMR spectrum shown below is formed. Identify the structure of both A and B. Suggest a synthesis for A starting from toluene, alcohols with 3 or fewer carbons and any other common reagents. Wavelength tim) 16 25 2 27 2 2 42 1200 3300 2200 2000 Wavemester teus Firger Print. Compound A IR spectrum Compound B H NMR A
Step by Step Solution
★★★★★
3.48 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Compound A gives the following IR spectrum Upon reaction with sodium ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6367e1d665310_237369.pdf
180 KBs PDF File
6367e1d665310_237369.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


