Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Consider the ion NCO- and the ion CNO. These ions have three resonance structures each that are NOT equivalent. Draw the Lewis structures
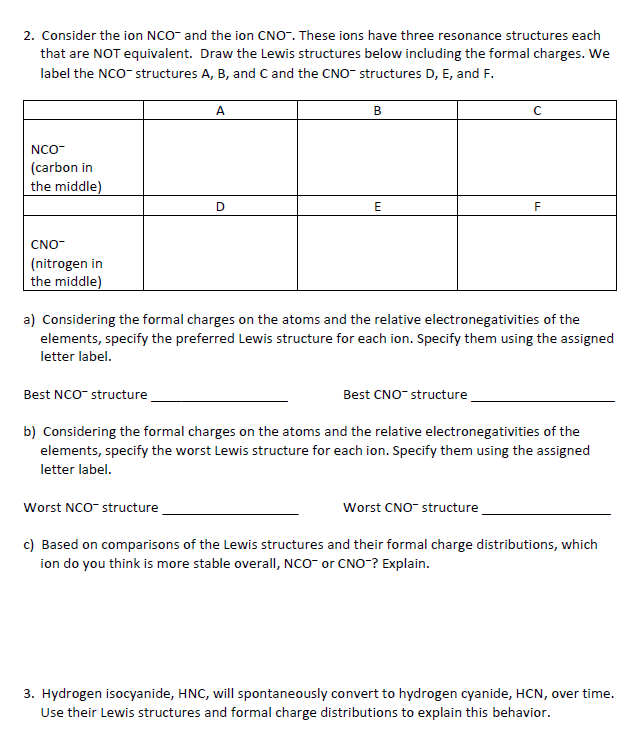
2. Consider the ion NCO- and the ion CNO. These ions have three resonance structures each that are NOT equivalent. Draw the Lewis structures below including the formal charges. We label the NCO-structures A, B, and C and the CNO- structures D, E, and F. NCO (carbon in the middle) CNO- (nitrogen in the middle) Best NCO-structure A D Worst NCO-structure B E a) Considering the formal charges on the atoms and the relative electronegativities of the elements, specify the preferred Lewis structure for each ion. Specify them using the assigned letter label. Best CNO structure C F b) Considering the formal charges on the atoms and the relative electronegativities of the elements, specify the worst Lewis structure for each ion. Specify them using the assigned letter label. Worst CNO- structure c) Based on comparisons of the Lewis structures and their formal charge distributions, which ion do you think is more stable overall, NCO or CNO? Explain. 3. Hydrogen isocyanide, HNC, will spontaneously convert to hydrogen cyanide, HCN, over time. Use their Lewis structures and formal charge distributions to explain this behavior.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer Solution NCO Carbon in the middle CN0 nitrogen in the middle Best b feloist borst A a Best N...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


