Question
2. Construct a hypothetical binary phase diagram of a system consisting S and T as components. Clearly labeled the X and Y axis, as
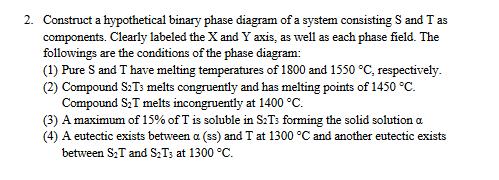
2. Construct a hypothetical binary phase diagram of a system consisting S and T as components. Clearly labeled the X and Y axis, as well as each phase field. The followings are the conditions of the phase diagram: (1) Pure S and T have melting temperatures of 1800 and 1550 C, respectively. (2) Compound S2T3 melts congruently and has melting points of 1450 C. Compound ST melts incongruently at 1400 C. (3) A maximum of 15% of T is soluble in S:Ts forming the solid solution a (4) A eutectic exists between a (ss) and T at 1300 C and another eutectic exists between S:T and ST3 at 1300 C.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



