Answered step by step
Verified Expert Solution
Question
1 Approved Answer
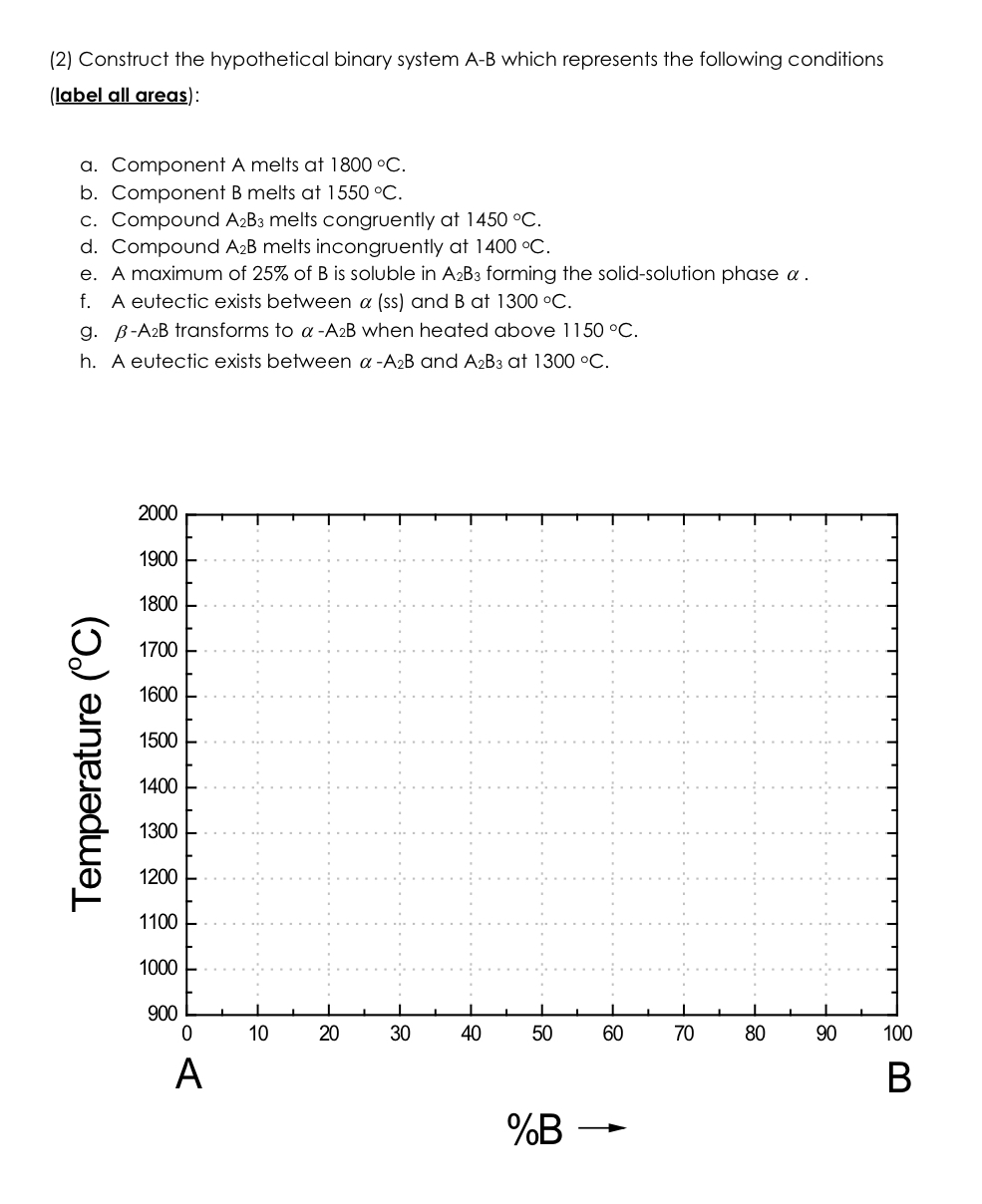
( 2 ) Construct the hypothetical binary system A - B which represents the following conditions ( label all areas ) : a . Component
Construct the hypothetical binary system AB which represents the following conditions
label all areas:
a Component A melts at
b Component melts at
c Compound melts congruently at
d Compound melts incongruently at
e A maximum of of is soluble in forming the solidsolution phase
f A eutectic exists between ss and at
g transforms to when heated above
h A eutectic exists between and at
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


