Question
2. Drug Development Onkology Pharmaceuticals is considering purchasing at most one of two competing drugs (molecules) developed by outside labs to beef up their oncology
2. Drug Development
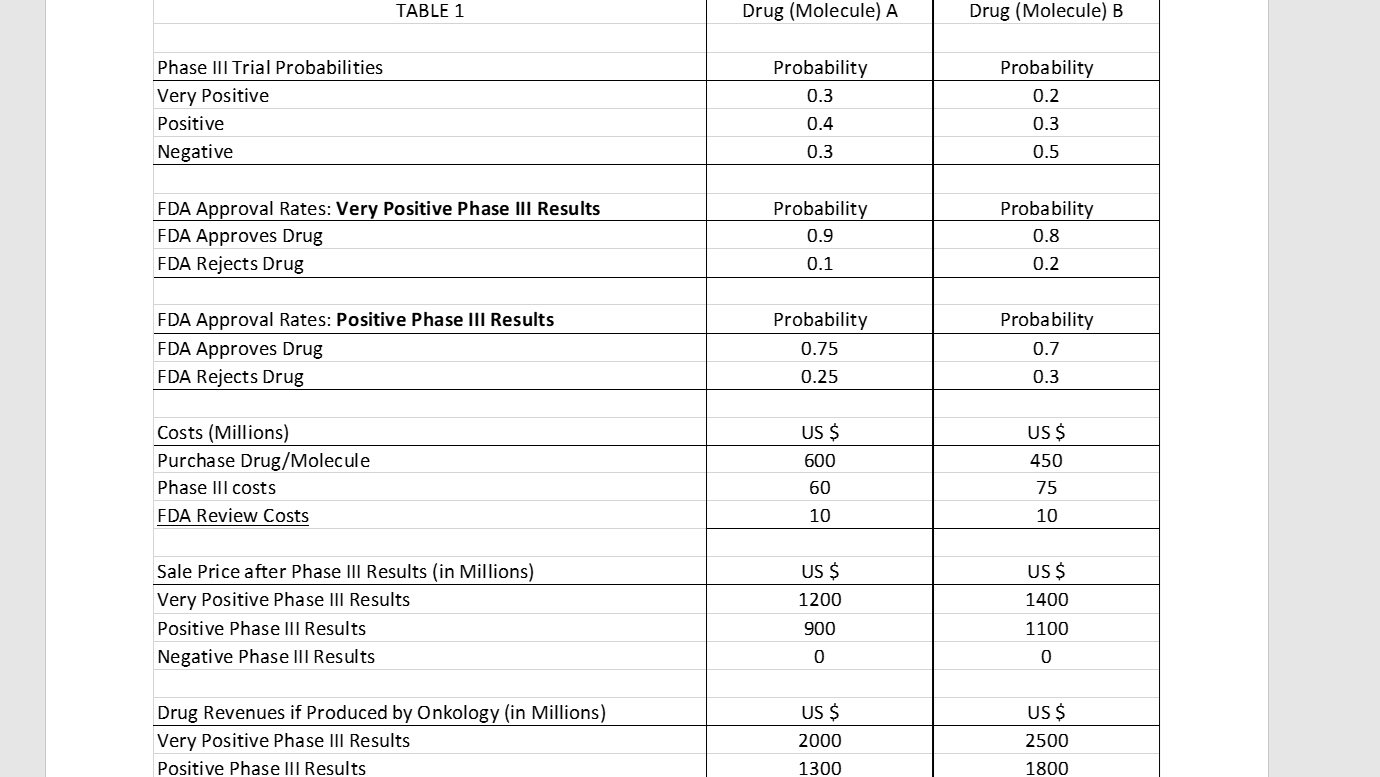
Onkology Pharmaceuticals is considering purchasing at most one of two competing drugs (molecules) developed by outside labs to beef up their oncology line. Drug A is from an existing class of drugs that has made it into the marketplace and is therefore considered less risky. Drug B would create a new class of molecules and therefore carries slightly more risk (and greater FDA scrutiny) but also has greater upside. Both drugs have successfully passed Phase II clinical trials, thus only a Phase III clinical trial (testing on humans) remains. If Onkology buys a drug, they will pay for Phase III testing.
The results of the Phase III trials can be classified as "very positive," "positive," or "negative." A negative Phase III result means the drug is worthless. If the results are very positive or positive, Onkology can either seek FDA approval or sell the drug to another company. If Onkology seeks FDA approval, the FDA can either approve or reject the drug. If the FDA approves the drug, Onkology will manufacture/sell the drug and collect all future revenues. If the FDA rejects the drug, Onkology loses their entire investment. The table below summarizes the probabilities, purchase costs, trial costs, sale prices, and future revenues.Assume all $ denominated quantities represent their present values.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


