Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Hot air humidifier (15 pt) A well-insulated humidifying unit is designed to lower the temperature of hot, dry air by bringing it into
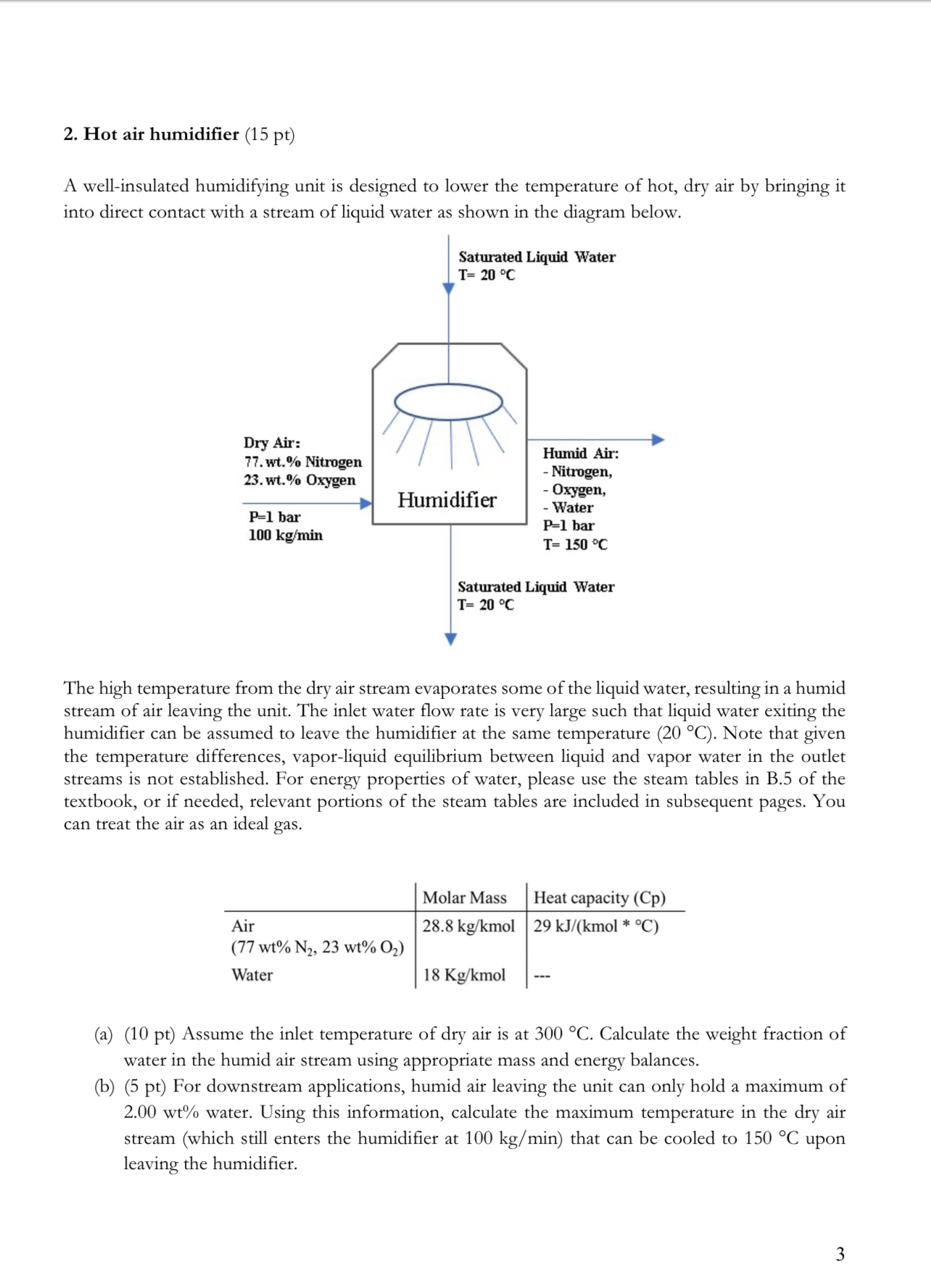
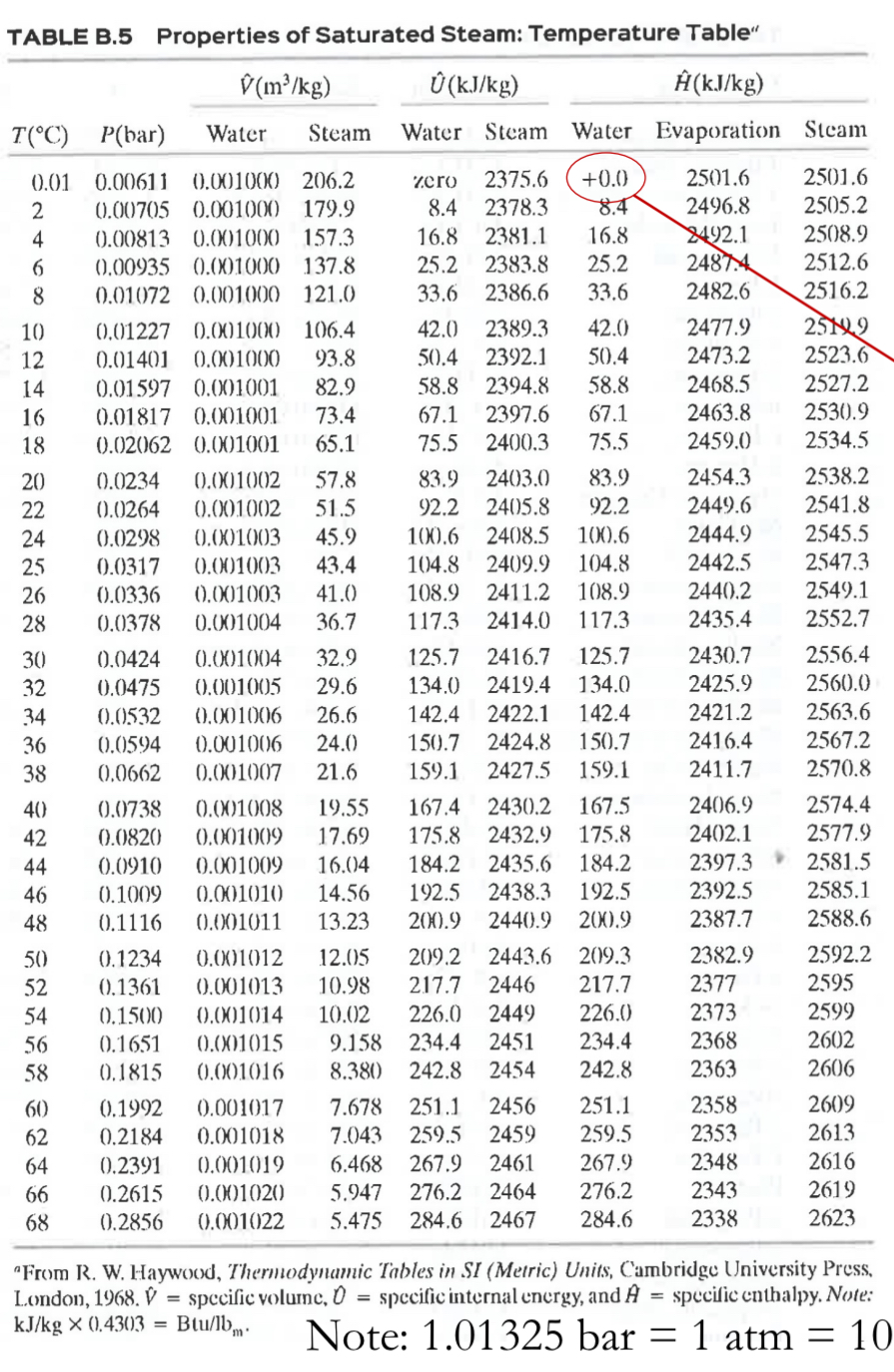
2. Hot air humidifier (15 pt) A well-insulated humidifying unit is designed to lower the temperature of hot, dry air by bringing it into direct contact with a stream of liquid water as shown in the diagram below. Saturated Liquid Water T= 20 C Dry Air: 77. wt.% Nitrogen 23. wt.% Oxygen P=1 bar 100 kg/min Humidifier Humid Air: - Nitrogen, - Oxygen, - Water P=1 bar T= 150 C Saturated Liquid Water T= 20 C The high temperature from the dry air stream evaporates some of the liquid water, resulting in a humid stream of air leaving the unit. The inlet water flow rate is very large such that liquid water exiting the humidifier can be assumed to leave the humidifier at the same temperature (20 C). Note that given the temperature differences, vapor-liquid equilibrium between liquid and vapor water in the outlet streams is not established. For energy properties of water, please use the steam tables in B.5 of the textbook, or if needed, relevant portions of the steam tables are included in subsequent pages. You can treat the air as an ideal gas. Molar Mass Heat capacity (Cp) 28.8 kg/kmol 29 kJ/(kmol * C) Air (77 wt% N2, 23 wt% O) Water 18 Kg/kmol (a) (10 pt) Assume the inlet temperature of dry air is at 300 C. Calculate the weight fraction of water in the humid air stream using appropriate mass and energy balances. (b) (5 pt) For downstream applications, humid air leaving the unit can only hold a maximum of 2.00 wt% water. Using this information, calculate the maximum temperature in the dry air stream (which still enters the humidifier at 100 kg/min) that can be cooled to 150 C upon leaving the humidifier. 3 TABLE B.5 Properties of Saturated Steam: Temperature Table" V(m/kg) (kJ/kg) (kJ/kg) T(C) P(bar) Water Steam Water Steam Water Evaporation Steam 2 0.01 0.00611 0.001000 206.2 0.00705 0.001000 179.9 zero 2375.6 +0.0 2501.6 2501.6 8.4 2378.3 8.4 2496.8 2505.2 4 0.00813 0.001000 157.3 16.8 2381.1 16.8 2492.1 2508.9 6 0.00935 0.001000 137.8 25.2 2383.8 25.2 2487.4 2512.6 8 0.01072 0.001000 121.0 33.6 2386.6 33.6 2482.6 2516.2 10 0.01227 0.001000 106.4 42.0 2389.3 42.0 2477.9 25199 20 211*2222222222332444 82TEMENTE 0.01401 0.001000 93.8 50.4 2392.1 50.4 2473.2 2523.6 0.01597 0.001001 82.9 58.8 2394.8 58.8 2468.5 2527.2 16 0.01817 0.001001 73.4 67.1 2397.6 67.1 2463.8 2530.9 18 0.02062 0.001001 65.1 75.5 2400.3 75.5 2459.0 2534.5 0.0234 0.001002 57.8 83.9 2403.0 83.9 2454.3 2538.2 25 0.0317 0.0264 0.0298 0.001003 45.9 0.001003 43.4 0.001002 51.5 92.2 2405.8 92.2 2449.6 2541.8 100.6 2408.5 100.6 2444.9 2545.5 104.8 2409.9 104.8 2442.5 2547.3 28 26 0.0336 0.0378 0.001003 41.0 108.9 2411.2 108.9 2440.2 2549.1 0.001004 36.7 117.3 2414.0 117.3 2435.4 2552.7 30 0.0424 0.001004 32.9 125.7 2416.7 125.7 2430.7 2556.4 0.0475 0.001005 29.6 134.0 2419.4 134.0 2425.9 2560.0 0.0532 0.001006 26.6 142.4 2422.1 142.4 2421.2 2563.6 36 0.0594 0.001006 24.0 150.7 2424.8 150.7 2416.4 2567.2 38 0.0662 0.001007 21.6 159.1 2427.5 159.1 2411.7 2570.8 40 0.0738 0.001008 19.55 167.4 2430.2 167.5 2406.9 2574.4 0.0820 0.001009 17.69 175.8 0.0910 0.001009 16.04 184.2 2435.6 184.2 2432.9 175.8 2402.1 2577.9 2397.3 2581.5 46 0.1009 0.001010 14.56 192.5 2438.3 192.5 2392.5 2585.1 48 0.1116 0.001011 13.23 200.9 2440.9 200.9 2387.7 2588.6 50 0.1234 0.001012 12.05 209.2 2443.6 209.3 2382.9 2592.2 52 0.1361 0.001013 10.98 217.7 2446 217.7 2377 2595 54 0.1500 0.001014 10.02 226.0 2449 226.0 2373 2599 56 0.1651 0.001015 9.158 234.4 2451 234.4 2368 2602 58 0.1815 0.001016 8.380 242.8 2454 242.8 2363 2606 60 0.1992 0.001017 7.678 251.1 2456 251.1 2358 2609 62 0.2184 0.001018 7.043 259.5 2459 259.5 2353 2613 64 0.2391 0.001019 6.468 267.9 2461 267.9 2348 2616 66 0.2615 0.001020 5.947 276.2 2464 276.2 2343 2619 68 0.2856 0.001022 5.475 284.6 2467 284.6 2338 2623 "From R. W. Haywood, Thermodynamic Tables in SI (Metric) Units, Cambridge University Press, London, 1968. V = specific volume, 0 specific internal energy, and A specific enthalpy. Note: = kJ/kg 0.4303 Btu/lbm. Note: 1.01325 bar = 1 atm = 10
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


