Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Hydrogen cyanide is prepared commercially by reacting methane and ammonia in the presence of oxygen over a platinum catalyst, as shown in the
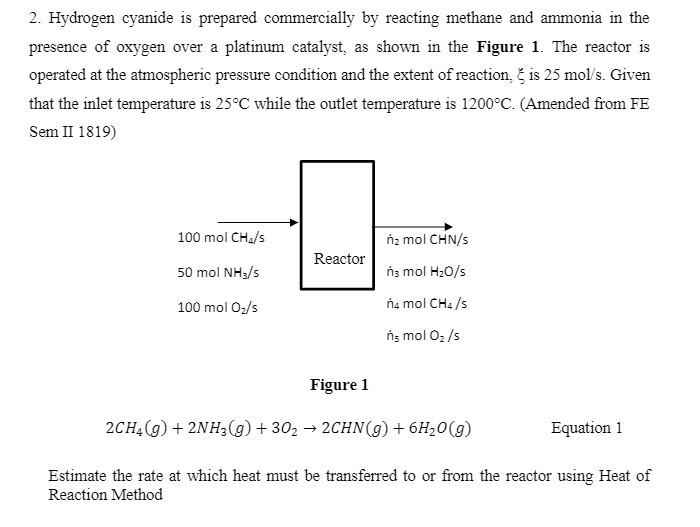
2. Hydrogen cyanide is prepared commercially by reacting methane and ammonia in the presence of oxygen over a platinum catalyst, as shown in the Figure 1. The reactor is operated at the atmospheric pressure condition and the extent of reaction, is 25 mol/s. Given that the inlet temperature is 25C while the outlet temperature is 1200C. (Amended from FE Sem II 1819) 100 mol CH4/s nz mol CHN/s Reactor 50 mol NH3/s n3 mol HO/s 100 mol O/s n4 mol CH4/s ns mol O/s Figure 1 2CH(g) + 2NH3(g) + 302 2CHN(g) + 6HO(g) Equation 1 Estimate the rate at which heat must be transferred to or from the reactor using Heat of Reaction Method
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


