2. Hydrogen is commercially made by the electrolysis of water 2H (aq) + 2e H2(g), Erev = 0.0 V SHE H2O(l) 1/2 O2(g) +
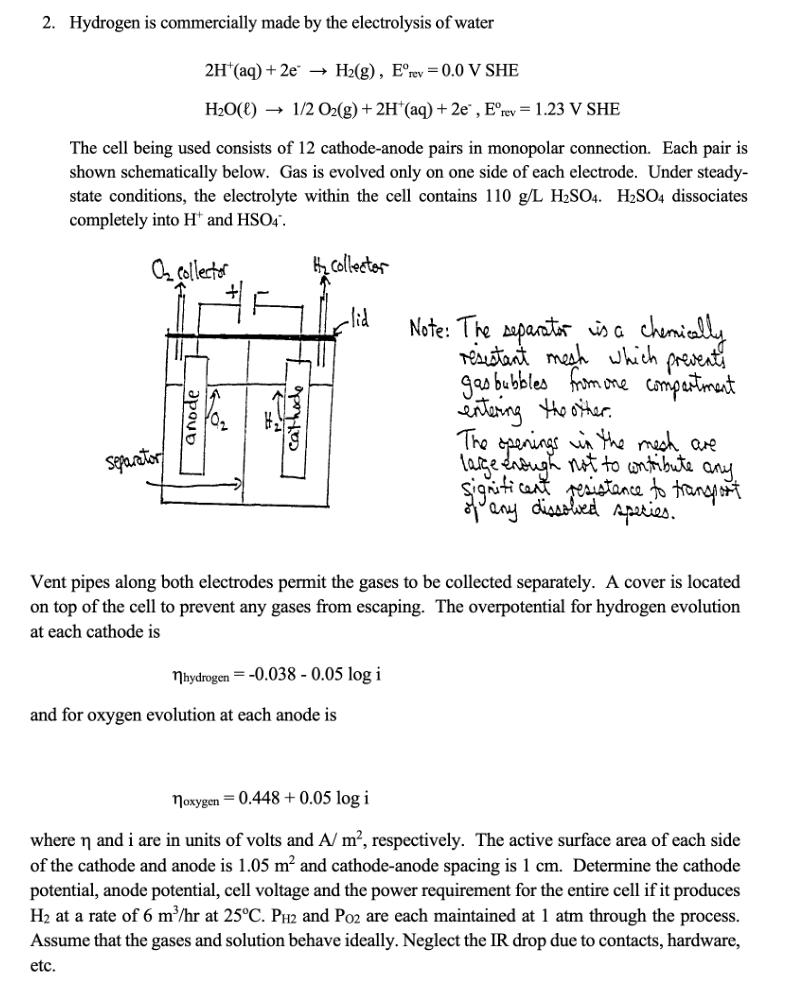
2. Hydrogen is commercially made by the electrolysis of water 2H (aq) + 2e H2(g), Erev = 0.0 V SHE H2O(l) 1/2 O2(g) + 2H(aq) + 2e, Erev = 1.23 V SHE The cell being used consists of 12 cathode-anode pairs in monopolar connection. Each pair is shown schematically below. Gas is evolved only on one side of each electrode. Under steady- state conditions, the electrolyte within the cell contains 110 g/L H2SO4. H2SO4 dissociates completely into H and HSO4. O collector H collector -lid Separator anode cathode Note: The separator is a chemically resistant mesh which prevents gas bubbles from one compartment entering the other. The openings in the mech are large enough not to contribute any Significant resistance to transport of any dissolved species. Vent pipes along both electrodes permit the gases to be collected separately. A cover is located on top of the cell to prevent any gases from escaping. The overpotential for hydrogen evolution at each cathode is nhydrogen -0.038 -0.05 log i and for oxygen evolution at each anode is noxygen 0.448+0.05 log i where n and i are in units of volts and A/ m, respectively. The active surface area of each side of the cathode and anode is 1.05 m and cathode-anode spacing is 1 cm. Determine the cathode potential, anode potential, cell voltage and the power requirement for the entire cell if it produces H2 at a rate of 6 m/hr at 25C. PH2 and P02 are each maintained at 1 atm through the process. Assume that the gases and solution behave ideally. Neglect the IR drop due to contacts, hardware, etc.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


