Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The experimental values i curretly have are: 1. The mass of the test tube= 15.9627g 2. Mass of lauric acid + test tube= 20.5718g 3.
The experimental values i curretly have are:
1. The mass of the test tube= 15.9627g
2. Mass of lauric acid + test tube= 20.5718g
3. mass of lauric acid= 4.6091g
4. Mass of lauric acid + benzonic acid + test tube 21.6319g
5. Mass of benzonic acid =1.1114g
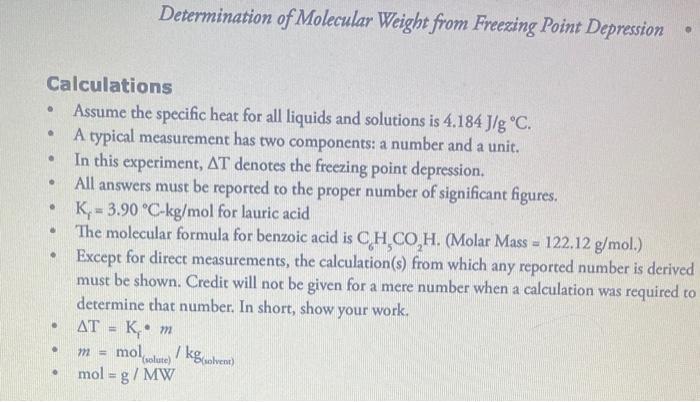

Calculations Assume the specific heat for all liquids and solutions is 4.184 J/g C. A typical measurement has two components: a number and a unit. . . . . Determination of Molecular Weight from Freezing Point Depression . In this experiment, AT denotes the freezing point depression. All answers must be reported to the proper number of significant figures. K-3.90 C-kg/mol for lauric acid The molecular formula for benzoic acid is CH,CO,H. (Molar Mass = 122.12 g/mol.) Except for direct measurements, the calculation(s) from which any reported number is derived must be shown. Credit will not be given for a mere number when a calculation was required to determine that number. In short, show your work. AT = K. m 772== mol, /kg(solvent) mol = g / MW (solute)
Step by Step Solution
★★★★★
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Ans6 freezing point depression AT ktm KF X WB X 1000 MB X WA Wg weight of solute 1...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


