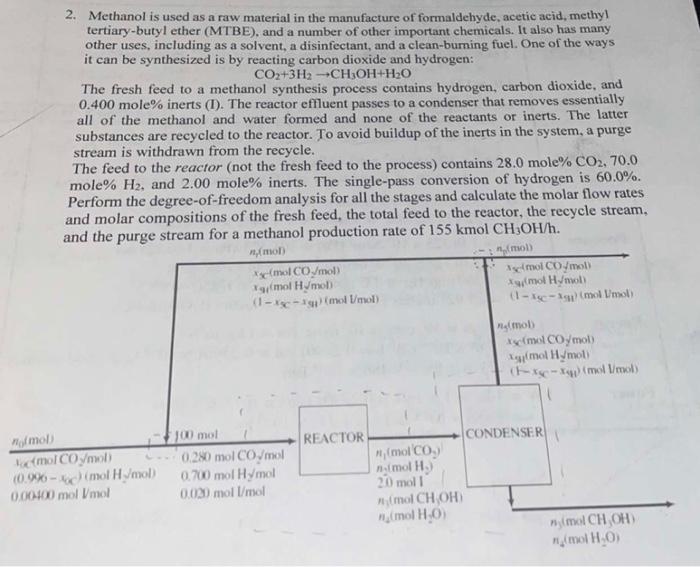
2. Methanol is used as a raw material in the manufacture of formaldehyde, acetic acid, methyl tertiary-butyl ether (MTBE), and a number of other important chemicals. It also has many other uses, including as a solvent, a disinfectant, and a clean-burning fuel. One of the ways it can be synthesized is by reacting carbon dioxide and hydrogen: CO2+3H2CH3OH+H2O The fresh feed to a methanol synthesis process contains hydrogen, carbon dioxide, and 0.400mole% inerts (I). The reactor effluent passes to a condenser that removes essentially all of the methanol and water formed and none of the reactants or inerts. The latter substances are recycled to the reactor. To avoid buildup of the inerts in the system, a purge stream is withdrawn from the recycle. The feed to the reactor (not the fresh feed to the process) contains 28.0mole%CO2,70.0 mole %H2, and 2.00 mole\% inerts. The single-pass conversion of hydrogen is 60.0%. Perform the degree-of-freedom analysis for all the stages and calculate the molar flow rates and molar compositions of the fresh feed, the total feed to the reactor, the recycle stream. and tha nurae stream for a methanol production rate of 155kmolCHCH3OH/h. 2. Methanol is used as a raw material in the manufacture of formaldehyde, acetic acid, methyl tertiary-butyl ether (MTBE), and a number of other important chemicals. It also has many other uses, including as a solvent, a disinfectant, and a clean-burning fuel. One of the ways it can be synthesized is by reacting carbon dioxide and hydrogen: CO2+3H2CH3OH+H2O The fresh feed to a methanol synthesis process contains hydrogen, carbon dioxide, and 0.400mole% inerts (I). The reactor effluent passes to a condenser that removes essentially all of the methanol and water formed and none of the reactants or inerts. The latter substances are recycled to the reactor. To avoid buildup of the inerts in the system, a purge stream is withdrawn from the recycle. The feed to the reactor (not the fresh feed to the process) contains 28.0mole%CO2,70.0 mole %H2, and 2.00 mole\% inerts. The single-pass conversion of hydrogen is 60.0%. Perform the degree-of-freedom analysis for all the stages and calculate the molar flow rates and molar compositions of the fresh feed, the total feed to the reactor, the recycle stream. and tha nurae stream for a methanol production rate of 155kmolCHCH3OH/h