Question
2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O at 298 K and 1 atm into HgO(s).
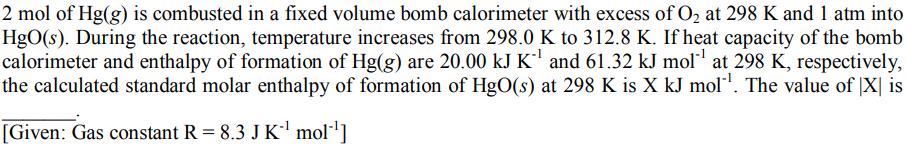
2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat capacity of the bomb calorimeter and enthalpy of formation of Hg(g) are 20.00 kJ K and 61.32 kJ mol at 298 K, respectively, the calculated standard molar enthalpy of formation of HgO(s) at 298 K is X kJ mol. The value of X is [Given: Gas constant R = 8.3 J K mol ]
Step by Step Solution
3.46 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



