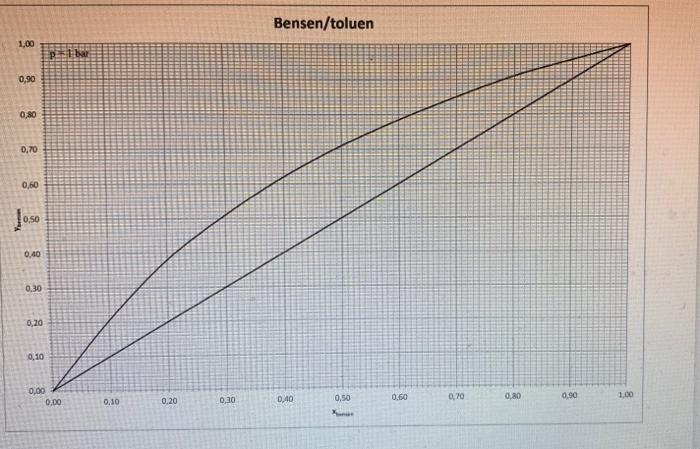
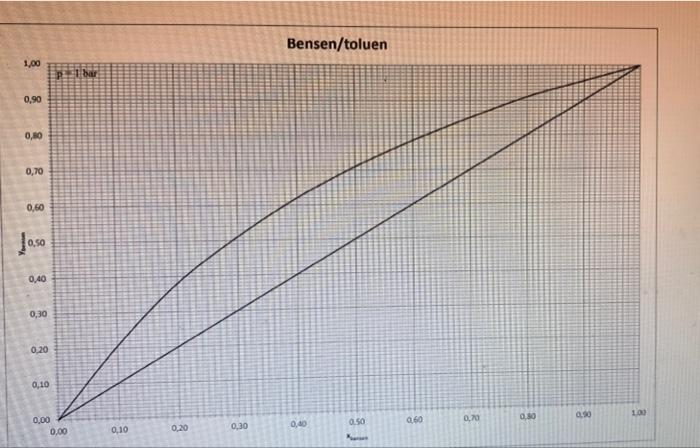
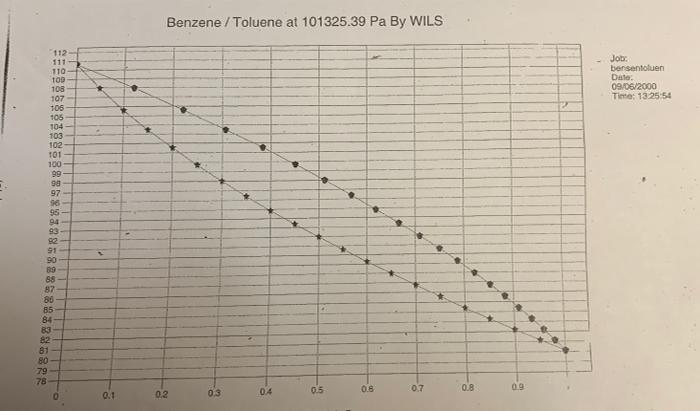
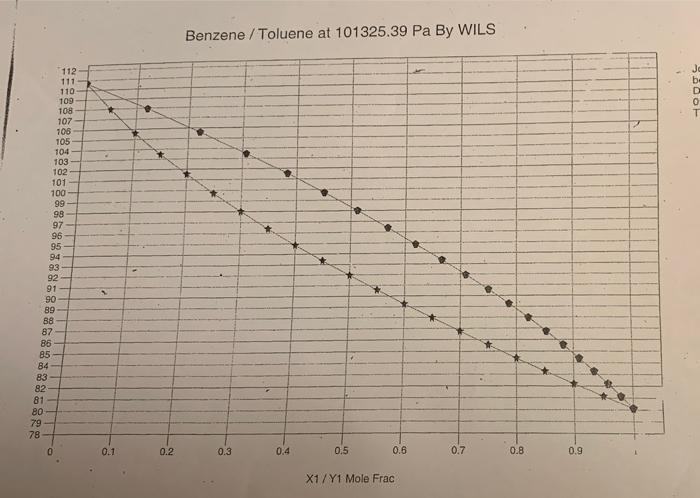
2. NOTE: For this task 2 there are three equilibrium diagrams and a temperature-content diagram attached. a) Determine the number of ideal bottoms required to separate a saturated steam containing 35 mol % benzene and the remainder toluene by distillation. The benzene content of distillates is 95 mol % and the bottom outlets may contain a maximum of 7 mol % benzene. When dimensioning, the reflux ratio R = 2 * Rmin must apply. Also calculate the column's internal steam flow above or below the inflow at the inflow 20 kmol / h. The column is equipped with a total condenser and reboiler and the separation is carried out at a pressure of 1 bar. b) Determine the temperature of the inflow using the attached temperature composition diagram. c) Assume that you are going to set up a heat balance over the column in (a) and you must therefore calculate the enthalpy for the inflow. Explain how you calculate this enthalpy and what physical data is required. The reference condition is 1 bar. 0C d) Someone claims that the separation requirement in (a) can be achieved with only four ideal bottoms. Is this theoretically possible? Your answer must be justified. 100 Tecken Stycke 2. NOTE: For this task 2 there are three equilibrium diagrams and a temperature-content diagram attached. a) Determine the number of ideal bottoms required to separate a saturated steam containing 35 mol % benzene and the remainder toluene by distillation. The benzene content of distillates is 95 mol % and the bottom outlets may contain a maximum of 7 mol% benzene. When dimensioning, the reflux ratio R = 2 * Rmin must apply. Also calculate the column's internal steam flow above or below the inflow at the inflow 20 kmol / h. The column is equipped with a total condenser and reboiler and the separation is carried out at a pressure of 1 bar. b) Determine the temperature of the inflow using the attached temperature composition diagram. Kmin must apply. Also calculate the column's internal ste flow above or below the inflow at the inflow 20 kmol/h. The column is equipped with a total condenser and reboiler and the separation is carried out at a pressure of 1 bar. b) Determine the temperature of the inflow using the attached temperature composition diagram. c) Assume that you are going to set up a heat balance over the column in (a) and you must therefore calculate the enthalpy for the inflow. Explain how you calculate this enthalpy and what physical data required. The reference condition is 1 bar. 0C d) Someone claims that the separation requirement in (a) can be achieved with only four ideal bottoms. Is this theoretically possible? Your answer must be justified. 1,00 0,90 0,80 0,70 0,60 0,50 0,40 0,30 0,20 0,10 0,00 p1 bar 0.00 0.10 0,20 0,30 Bensen/toluen 0,40 0.50 Xun 0.60 0,70 0,80 0,90 1,00 1,00 0,90 0,80 0,70 0,60 10.50 0,40 0,30 0,20 0,10 0,00 p+1 bar 0,00 0,10 0,20 0,30 Bensen/toluen 0,40 0.50 X 090 0,70 0,80 0670 1.00 112- 111 110- 100 108 107 106 105 104 103 102 101 100 99 98 97 96 95 94 93 92 91 90 89 88 87 85 85 84 83 82 81 80 79 78 0.1 Benzene / Toluene at 101325.39 Pa By WILS 04 0.5 0.8 0.7 0.2 0.3 0.8 0.9 Job: bersentoluen Date: 09/06/2000 Time: 13:25:54 112 111 110 109 108 107 106 105 104 103 102 101 100 99 98 97 96 95 94 93 92 91 90 89 88 87 86 85 84 83 82 81 80 79 78 0 0.1 0.2 Benzene / Toluene at 101325.39 Pa By WILS 0.3 0.4 . 0.5 X1/Y1 Mole Frac 0.6 0.7 0.8 0.9 2. NOTE: For this task 2 there are three equilibrium diagrams and a temperature-content diagram attached. a) Determine the number of ideal bottoms required to separate a saturated steam containing 35 mol % benzene and the remainder toluene by distillation. The benzene content of distillates is 95 mol % and the bottom outlets may contain a maximum of 7 mol % benzene. When dimensioning, the reflux ratio R = 2 * Rmin must apply. Also calculate the column's internal steam flow above or below the inflow at the inflow 20 kmol / h. The column is equipped with a total condenser and reboiler and the separation is carried out at a pressure of 1 bar. b) Determine the temperature of the inflow using the attached temperature composition diagram. c) Assume that you are going to set up a heat balance over the column in (a) and you must therefore calculate the enthalpy for the inflow. Explain how you calculate this enthalpy and what physical data is required. The reference condition is 1 bar. 0C d) Someone claims that the separation requirement in (a) can be achieved with only four ideal bottoms. Is this theoretically possible? Your answer must be justified. 100 Tecken Stycke 2. NOTE: For this task 2 there are three equilibrium diagrams and a temperature-content diagram attached. a) Determine the number of ideal bottoms required to separate a saturated steam containing 35 mol % benzene and the remainder toluene by distillation. The benzene content of distillates is 95 mol % and the bottom outlets may contain a maximum of 7 mol% benzene. When dimensioning, the reflux ratio R = 2 * Rmin must apply. Also calculate the column's internal steam flow above or below the inflow at the inflow 20 kmol / h. The column is equipped with a total condenser and reboiler and the separation is carried out at a pressure of 1 bar. b) Determine the temperature of the inflow using the attached temperature composition diagram. Kmin must apply. Also calculate the column's internal ste flow above or below the inflow at the inflow 20 kmol/h. The column is equipped with a total condenser and reboiler and the separation is carried out at a pressure of 1 bar. b) Determine the temperature of the inflow using the attached temperature composition diagram. c) Assume that you are going to set up a heat balance over the column in (a) and you must therefore calculate the enthalpy for the inflow. Explain how you calculate this enthalpy and what physical data required. The reference condition is 1 bar. 0C d) Someone claims that the separation requirement in (a) can be achieved with only four ideal bottoms. Is this theoretically possible? Your answer must be justified. 1,00 0,90 0,80 0,70 0,60 0,50 0,40 0,30 0,20 0,10 0,00 p1 bar 0.00 0.10 0,20 0,30 Bensen/toluen 0,40 0.50 Xun 0.60 0,70 0,80 0,90 1,00 1,00 0,90 0,80 0,70 0,60 10.50 0,40 0,30 0,20 0,10 0,00 p+1 bar 0,00 0,10 0,20 0,30 Bensen/toluen 0,40 0.50 X 090 0,70 0,80 0670 1.00 112- 111 110- 100 108 107 106 105 104 103 102 101 100 99 98 97 96 95 94 93 92 91 90 89 88 87 85 85 84 83 82 81 80 79 78 0.1 Benzene / Toluene at 101325.39 Pa By WILS 04 0.5 0.8 0.7 0.2 0.3 0.8 0.9 Job: bersentoluen Date: 09/06/2000 Time: 13:25:54 112 111 110 109 108 107 106 105 104 103 102 101 100 99 98 97 96 95 94 93 92 91 90 89 88 87 86 85 84 83 82 81 80 79 78 0 0.1 0.2 Benzene / Toluene at 101325.39 Pa By WILS 0.3 0.4 . 0.5 X1/Y1 Mole Frac 0.6 0.7 0.8 0.9