Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Provide a mechanism for the following transformation. Make sure to account for the formation of this particular diastereomer by paying careful attention to
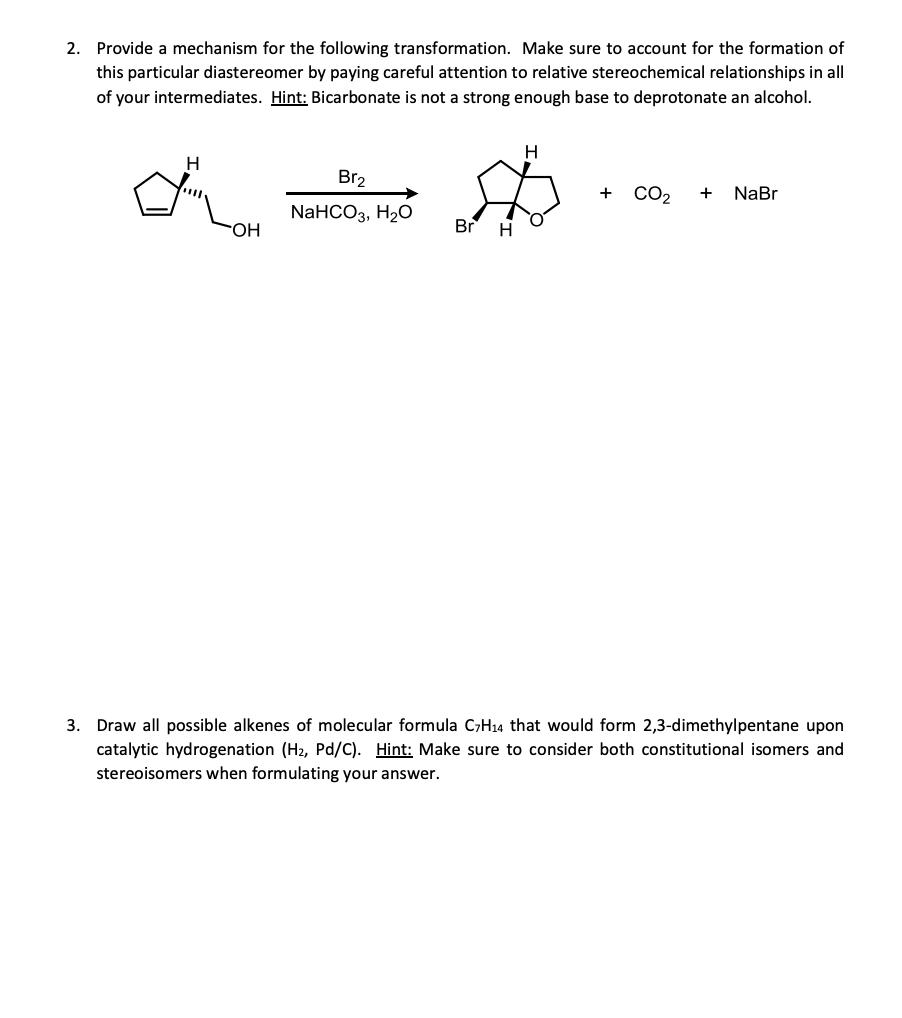
2. Provide a mechanism for the following transformation. Make sure to account for the formation of this particular diastereomer by paying careful attention to relative stereochemical relationships in all of your intermediates. Hint: Bicarbonate is not a strong enough base to deprotonate an alcohol. H OH Br NaHCO3, HO Br H H + CO + NaBr 3. Draw all possible alkenes of molecular formula C7H4 that would form 2,3-dimethylpentane upon catalytic hydrogenation (H, Pd/C). Hint: Make sure to consider both constitutional isomers and stereoisomers when formulating your answer.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
as 2 tr A Caba CH Hwyder FF n 0k a ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


