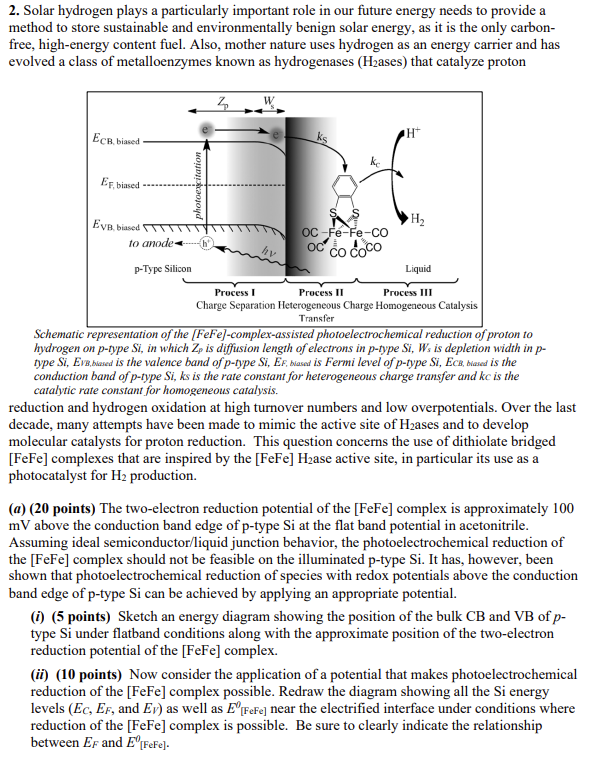
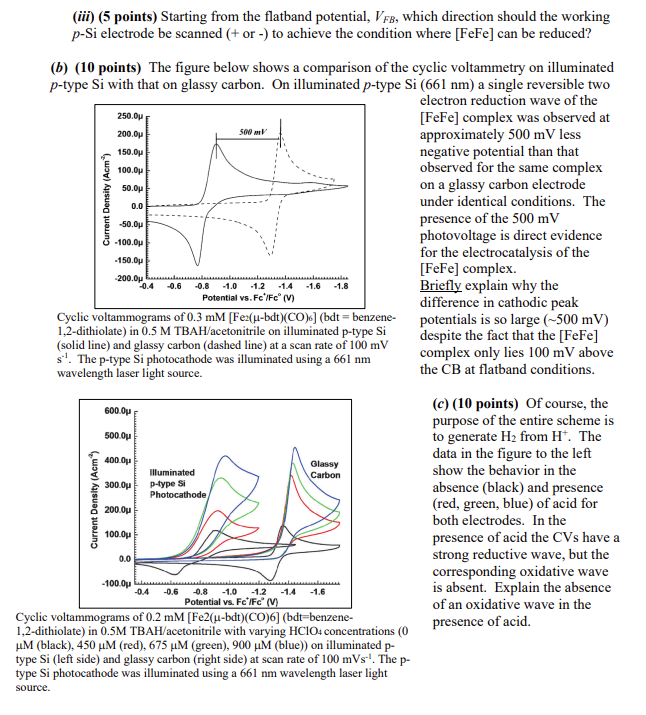
2. Solar hydrogen plays a particularly important role in our future energy needs to provide a method to store sustainable and environmentally benign solar energy, as it is the only carbonfree, high-energy content fuel. Also, mother nature uses hydrogen as an energy carrier and has evolved a class of metalloenzymes known as hydrogenases ( H2 ases) that catalyze proton Schematic representation of the [FeFe]-complex-assisted photoelectrochemical reduction of proton to hydrogen on p-type Si, in which Zp is diffusion length of electrons in p-type Si,Ws is depletion width in ptype Si, Evr,biased is the valence band of p-type Si, EF, biasal is Fermi level of p-type Si, ECB, biased is the conduction band of p-type Si, ks is the rate constant for heterogeneous charge transfer and kc is the catalytic rate constant for homogeneous catalysis. reduction and hydrogen oxidation at high turnover numbers and low overpotentials. Over the last decade, many attempts have been made to mimic the active site of H2 ases and to develop molecular catalysts for proton reduction. This question concerns the use of dithiolate bridged [FeFe] complexes that are inspired by the [FeFe]H2 ase active site, in particular its use as a photocatalyst for H2 production. (a) (20 points) The two-electron reduction potential of the [FeFe] complex is approximately 100 mV above the conduction band edge of p-type Si at the flat band potential in acetonitrile. Assuming ideal semiconductor/liquid junction behavior, the photoelectrochemical reduction of the [FeFe] complex should not be feasible on the illuminated p-type Si. It has, however, been shown that photoelectrochemical reduction of species with redox potentials above the conduction band edge of p-type Si can be achieved by applying an appropriate potential. (i) (5 points) Sketch an energy diagram showing the position of the bulk CB and VB of p type Si under flatband conditions along with the approximate position of the two-electron reduction potential of the [FeFe] complex. (ii) (10 points) Now consider the application of a potential that makes photoelectrochemical reduction of the [FeFe] complex possible. Redraw the diagram showing all the Si energy levels (EC,EF, and EV) as well as E[FeFe]0 near the electrified interface under conditions where reduction of the [FeFe] complex is possible. Be sure to clearly indicate the relationship between EF and E0[FeFe]. (iii) (5 points) Starting from the flatband potential, VFB, which direction should the working p-Si electrode be scanned ( + or -) to achieve the condition where [FeFe] can be reduced? (b) (10 points) The figure below shows a comparison of the cyclic voltammetry on illuminated p-type Si with that on glassy carbon. On illuminated p-type Si(661nm) a single reversible two electron reduction wave of the [FeFe] complex was observed at approximately 500mV less negative potential than that observed for the same complex on a glassy carbon electrode under identical conditions. The presence of the 500mV photovoltage is direct evidence for the electrocatalysis of the [FeFe] complex. Briefly explain why the difference in cathodic peak Cyclic voltammograms of 0.3mM[Fe2(bdt)(CO)6] (bdt = benzene- potentials is so large (500mV) 1,2 -dithiolate) in 0.5MTBAH/ acetonitrile on illuminated p-type Si despite the fact that the [FeFe] (solidline)andglassycarbon(dashedline)atascanrateof100mVs1.Thep-typeSiphotocathodewasilluminatedusinga661nmdespitethefactthatcomplexonlylies100mVabove wavelength laser light source. the CB at flatband conditions. (c) (10 points) Of course, the purpose of the entire scheme is to generate H2 from H+. The data in the figure to the left show the behavior in the absence (black) and presence (red, green, blue) of acid for both electrodes. In the presence of acid the CVs have a strong reductive wave, but the corresponding oxidative wave is absent. Explain the absence of an oxidative wave in the M (black), 450M (red), 675M (green), 900M (blue)) on illuminated ptype Si (left side) and glassy carbon (right side) at scan rate of 100mVs1. The ptype Si photocathode was illuminated using a 661nm wavelength laser light source








