Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. The decomposition of acetaldehyde (CH3CHO) to methane and carbon monoxide is an example of a free radical chain reaction. The overall reaction is believed
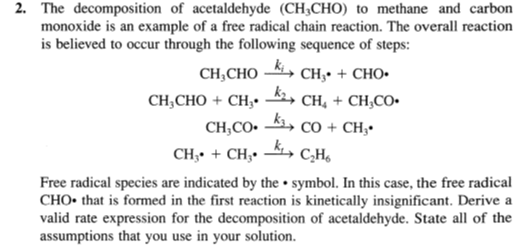 2. The decomposition of acetaldehyde (CH3CHO) to methane and carbon monoxide is an example of a free radical chain reaction. The overall reaction is believed to occur through the following sequence of steps: CH3CHOkiCH3+CHOCH3CHO+CH3k2CH4+CH3COCH3COk3CO+CH3CH3+CH3k1C2H6 Free radical species are indicated by the symbol. In this case, the free radical CHO that is formed in the first reaction is kinetically insignificant. Derive a valid rate expression for the decomposition of acetaldehyde. State all of the assumptions that you use in your solution
2. The decomposition of acetaldehyde (CH3CHO) to methane and carbon monoxide is an example of a free radical chain reaction. The overall reaction is believed to occur through the following sequence of steps: CH3CHOkiCH3+CHOCH3CHO+CH3k2CH4+CH3COCH3COk3CO+CH3CH3+CH3k1C2H6 Free radical species are indicated by the symbol. In this case, the free radical CHO that is formed in the first reaction is kinetically insignificant. Derive a valid rate expression for the decomposition of acetaldehyde. State all of the assumptions that you use in your solution Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


