Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. The Eh that a half reaction couple generates is generally weakly sensitive to the concentrations of the reactants and products involved, including pH
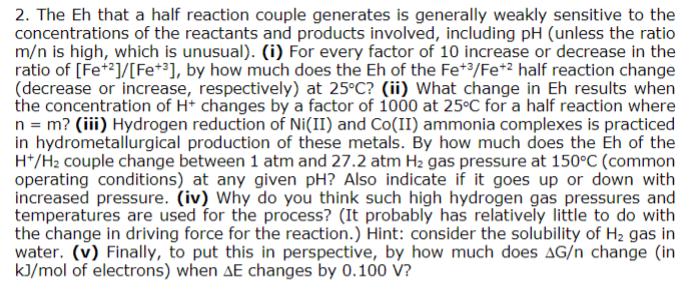
2. The Eh that a half reaction couple generates is generally weakly sensitive to the concentrations of the reactants and products involved, including pH (unless the ratio m/n is high, which is unusual). (i) For every factor of 10 increase or decrease in the ratio of [Fe+2]/[Fe+3], by how much does the Eh of the Fe+3/Fe+2 half reaction change (decrease or increase, respectively) at 25C? (ii) What change in Eh results when the concentration of H+ changes by a factor of 1000 at 25C for a half reaction where n = m? (iii) Hydrogen reduction of Ni(II) and Co(II) ammonia complexes is practiced in hydrometallurgical production of these metals. By how much does the Eh of the H+/H2 couple change between 1 atm and 27.2 atm H2 gas pressure at 150C (common operating conditions) at any given pH? Also indicate if it goes up or down with increased pressure. (iv) Why do you think such high hydrogen gas pressures and temperatures are used for the process? (It probably has relatively little to do with the change in driving force for the reaction.) Hint: consider the solubility of H gas in water. (v) Finally, to put this in perspective, by how much does AG/n change (in kJ/mol of electrons) when AE changes by 0.100 V?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


