Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. The elementary irreversible liquid-phase reaction of A+ B C dm mol.min' is carried out with the reaction rate constant of k = 0.5
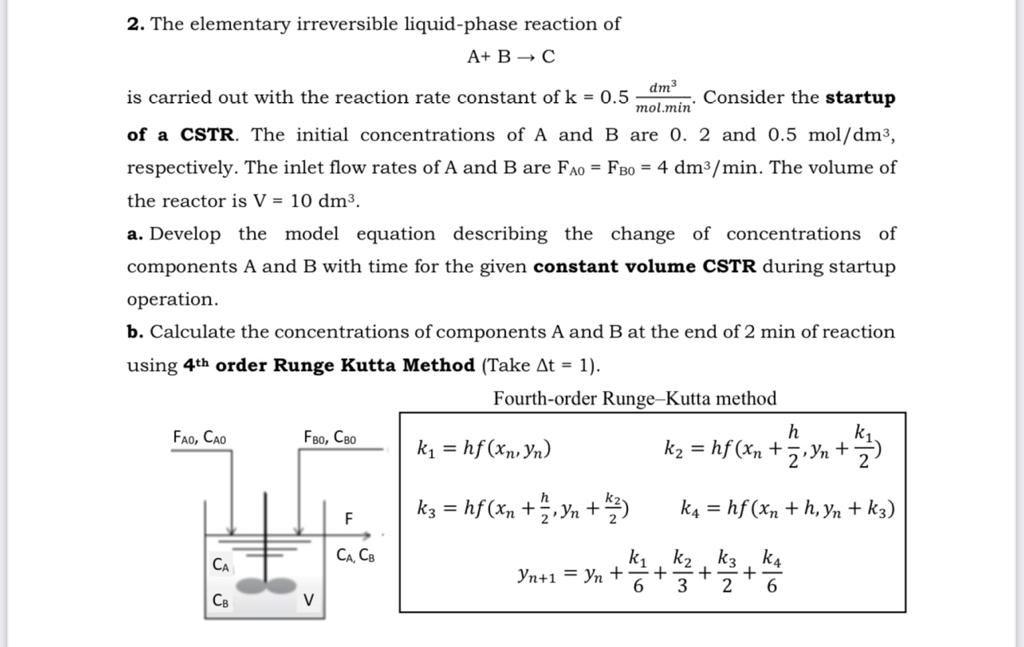
2. The elementary irreversible liquid-phase reaction of A+ B C dm mol.min' is carried out with the reaction rate constant of k = 0.5 Consider the startup of a CSTR. The initial concentrations of A and B are 0. 2 and 0.5 mol/dm, respectively. The inlet flow rates of A and B are FAO = FBO = 4 dm/min. The volume of the reactor is V = 10 dm. a. Develop the model equation describing the change of concentrations of components A and B with time for the given constant volume CSTR during startup operation. b. Calculate the concentrations of components A and B at the end of 2 min of reaction using 4th order Runge Kutta Method (Take At = 1). Fourth-order Runge-Kutta method FAO, CAO CB FBO, CBO V F CA, CB k = hf (xn, Yn) K3 = hf (xn+ h 27 Yn + 1/27) h k = hf (xn + , Yn + /) 2 k4 = hf (xn + h, Yn + k3) k k k3 K4 Yn+1=Yn + + + + 6 3 2 6
Step by Step Solution
★★★★★
3.34 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Here are the steps to solve this problem using the 4th order Ru...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


