Question
The van-der Waals equation is (p+)(V-b)= RT, where P is the pressure, V the molar volume, and T is the temperature of the given
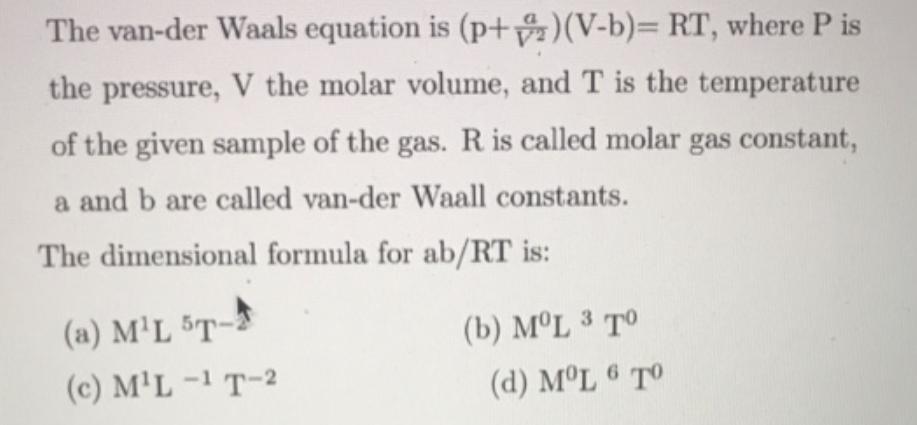
The van-der Waals equation is (p+)(V-b)= RT, where P is the pressure, V the molar volume, and T is the temperature of the given sample of the gas. R is called molar gas constant, a and b are called van-der Waall constants. The dimensional formula for ab/RT is: (a) M'L 5T- (b) ML 3 T (c) M'L -1 T-2 (d) ML 6 TO
Step by Step Solution
3.42 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Matlab An Introduction with Applications
Authors: Amos Gilat
5th edition
1118629868, 978-1118801802, 1118801806, 978-1118629864
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



