Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2.) The reaction AD, takes place in a constant volume CSTR. The volume is held at 5 L and the inlet concentration is found
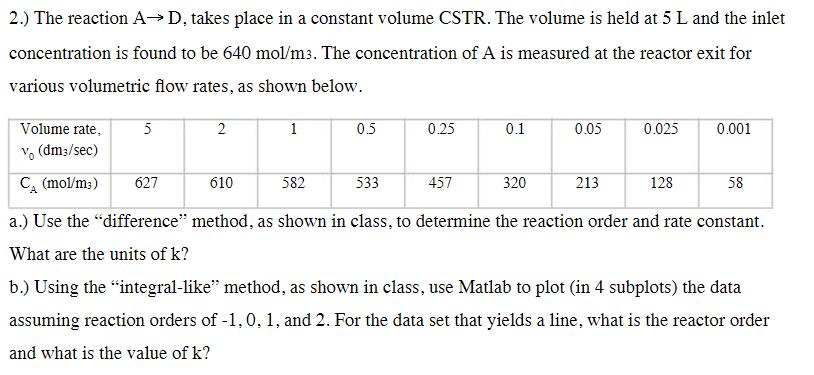
2.) The reaction AD, takes place in a constant volume CSTR. The volume is held at 5 L and the inlet concentration is found to be 640 mol/m3. The concentration of A is measured at the reactor exit for various volumetric flow rates, as shown below. Volume rate, vo (dm3/sec) 5 2 1 05 0.25 0.1 0.05 0.025 0.001 CA (mol/m3) 627 610 582 533 457 320 213 128 58 a.) Use the "difference" method, as shown in class, to determine the reaction order and rate constant. What are the units of k? b.) Using the "integral-like" method, as shown in class, use Matlab to plot (in 4 subplots) the data assuming reaction orders of -1, 0, 1, and 2. For the data set that yields a line, what is the reactor order and what is the value of k?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Using the difference method The general rate equation for a constant volume CSTR is r kCAn where r ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


