Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. The reaction is given below at constant temperature in a continuous stirred tank reactor: k A+B+C+D -1A = (6h-)CA [kmol/mh] The concentrations of
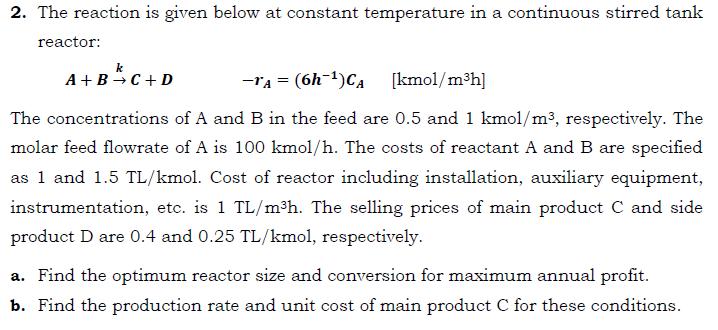
2. The reaction is given below at constant temperature in a continuous stirred tank reactor: k A+B+C+D -1A = (6h-)CA [kmol/mh] The concentrations of A and B in the feed are 0.5 and 1 kmol/m, respectively. The molar feed flowrate of A is 100 kmol/h. The costs of reactant A and B are specified as 1 and 1.5 TL/kmol. Cost of reactor including installation, auxiliary equipment, instrumentation, etc. is 1 TL/mh. The selling prices of main product C and side product D are 0.4 and 0.25 TL/kmol, respectively. a. Find the optimum reactor size and conversion for maximum annual profit. b. Find the production rate and unit cost of main product C for these conditions.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
A chemical engineering problem Lets break it down step by step Part a Find the optimum reactor size and conversion for maximum annual profit We need t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
66428adb19a6d_979331.pdf
180 KBs PDF File
66428adb19a6d_979331.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


