Question
2. Water Molecule A water molecule consists of two hydrogen atoms (each of mass 1.008 u) bonded to an oxygen atom (of mass 16.00
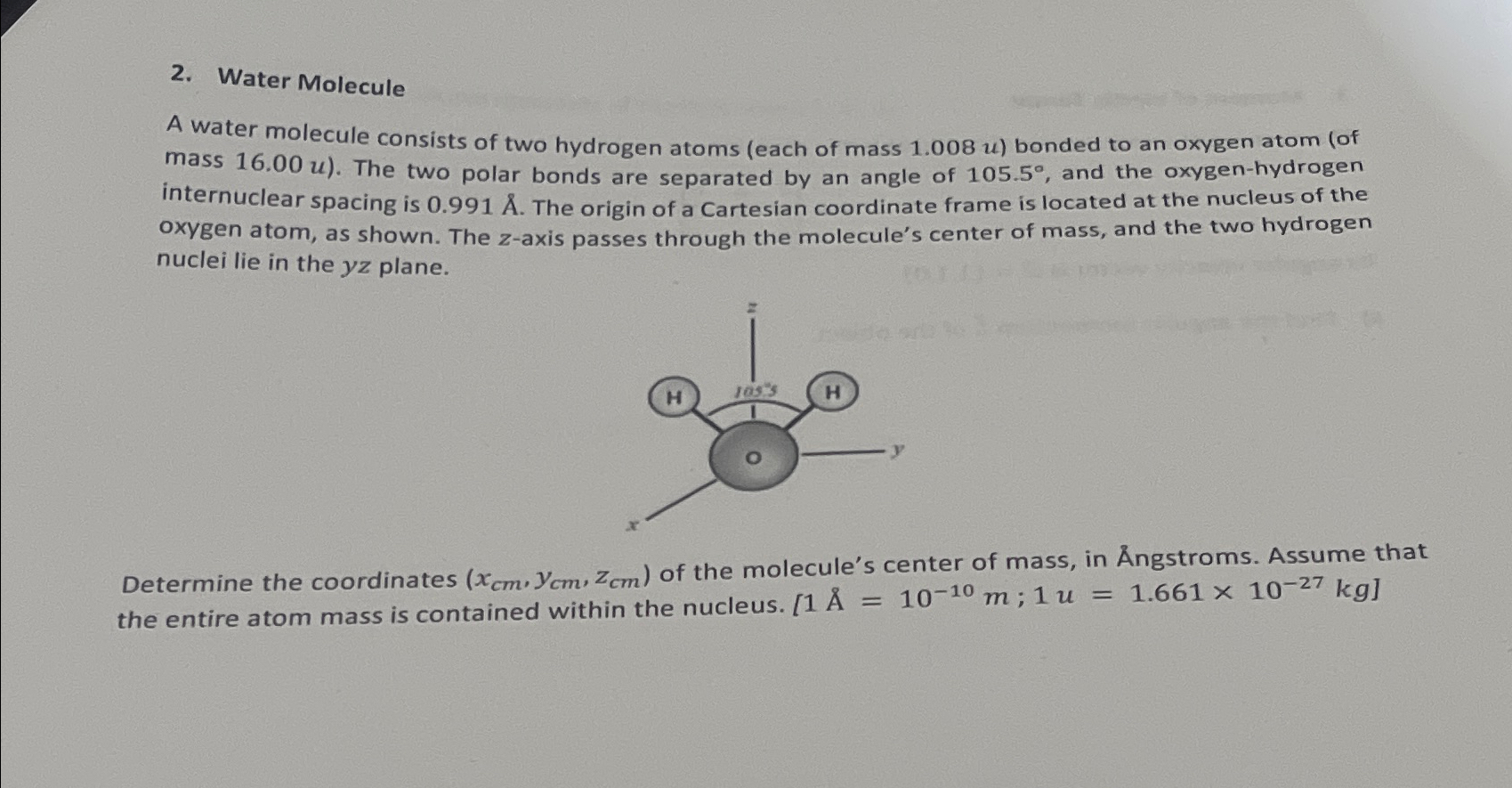
2. Water Molecule A water molecule consists of two hydrogen atoms (each of mass 1.008 u) bonded to an oxygen atom (of mass 16.00 u). The two polar bonds are separated by an angle of 105.5, and the oxygen-hydrogen internuclear spacing is 0.991 . The origin of a Cartesian coordinate frame is located at the nucleus of the oxygen atom, as shown. The z-axis passes through the molecule's center of mass, and the two hydrogen nuclei lie in the yz plane. H 105's H y Determine the coordinates (xcm. Yem, Zem) of the molecule's center of mass, in ngstroms. Assume that the entire atom mass is contained within the nucleus. [1 = 10-10 m; 1 u = 1.661 x 10-27 kg]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Linear Algebra
Authors: Peter J. Olver, Cheri Shakiban
1st edition
131473824, 978-0131473829
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



