Question
2. You are tasked to use a strong-acid polystyrene cross-linked with pure divinyl benzene ion- exchanger resin operated with a sulfonated sodium to remove
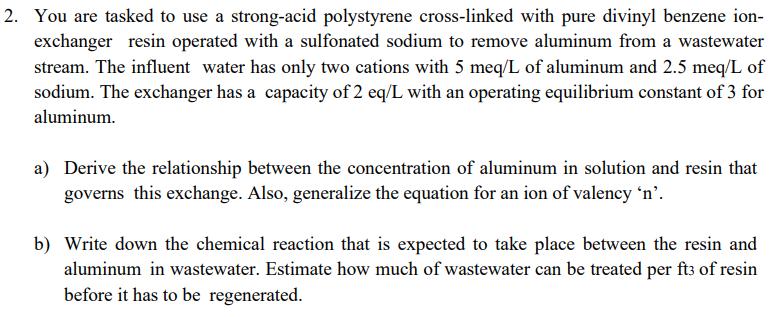
2. You are tasked to use a strong-acid polystyrene cross-linked with pure divinyl benzene ion- exchanger resin operated with a sulfonated sodium to remove aluminum from a wastewater stream. The influent water has only two cations with 5 meq/L of aluminum and 2.5 meq/L of sodium. The exchanger has a capacity of 2 eq/L with an operating equilibrium constant of 3 for aluminum. a) Derive the relationship between the concentration of aluminum in solution and resin that governs this exchange. Also, generalize the equation for an ion of valency 'n'. b) Write down the chemical reaction that is expected to take place between the resin and aluminum in wastewater. Estimate how much of wastewater can be treated per ft3 of resin before it has to be regenerated.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App