Question
2. You are tasked with identifying a piece of metal that has either a BCC or FCC structure. The following x-ray diffraction spectra using
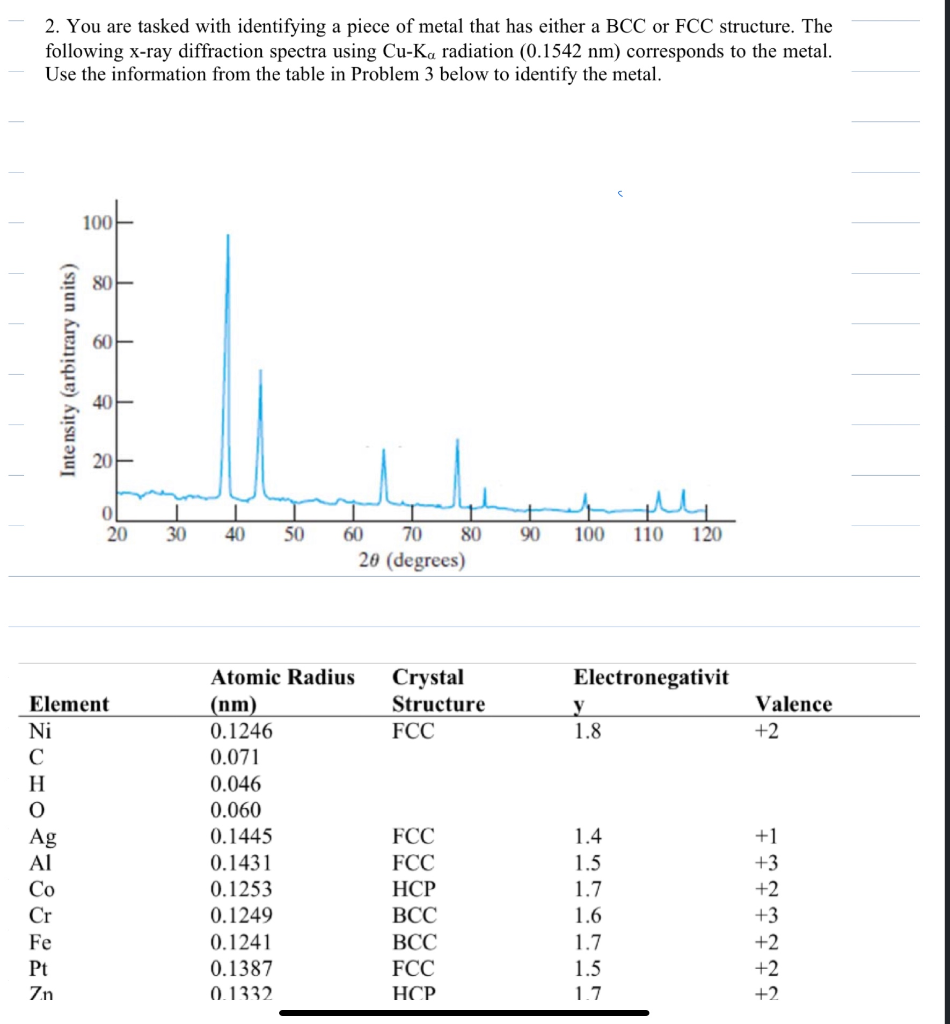
2. You are tasked with identifying a piece of metal that has either a BCC or FCC structure. The following x-ray diffraction spectra using Cu-Ka radiation (0.1542 nm) corresponds to the metal. Use the information from the table in Problem 3 below to identify the metal. 100 Intensity (arbitrary units) 8 40H 60H 80 0 20 30 40 50 60 70 80 90 100 110 20 (degrees) 120 Atomic Radius Crystal Electronegativit Element (nm) Structure y Ni 0.1246 FCC 1.8 Valence +2 CHONOURNE 0.071 0.046 0.060 Ag 0.1445 FCC 1.4 +1 0.1431 FCC 1.5 +3 Co 0.1253 HCP 1.7 +2 Cr 0.1249 BCC 1.6 +3 Fe 0.1241 BCC 1.7 +2 Pt 0.1387 FCC 1.5 +2 Zn 0.1332 HCP 1.7 +2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics For Managers Using Microsoft Excel
Authors: David M. Levine, David F. Stephan, Kathryn A. Szabat
7th Edition
978-0133061819, 133061817, 978-0133130805
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



