Question
20. 21. ds Starting from the definitions of temperature = and heat capacity C = following identities: a) C = Tds prove the ds
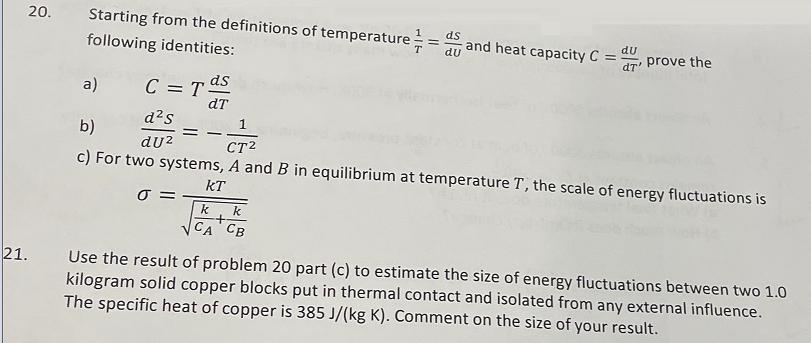
20. 21. ds Starting from the definitions of temperature = and heat capacity C = following identities: a) C = Tds prove the ds b) du dT 1 CT2 c) For two systems, A and B in equilibrium at temperature T, the scale of energy fluctuations is KT k k + CA CB Use the result of problem 20 part (c) to estimate the size of energy fluctuations between two 1.0 kilogram solid copper blocks put in thermal contact and isolated from any external influence. The specific heat of copper is 385 J/(kg K). Comment on the size of your result.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus Of A Single Variable
Authors: Ron Larson, Bruce H. Edwards
11th Edition
978-1337275361, 9781337275361
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



