Question: 20. Before it can be fed to the gasification reactor, coal is ground to meet specifications on particle size. Since the ratio of dry coal

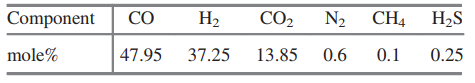

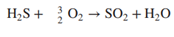


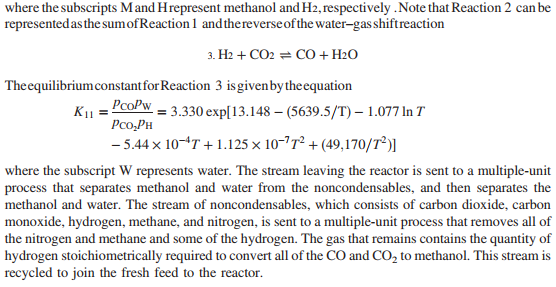
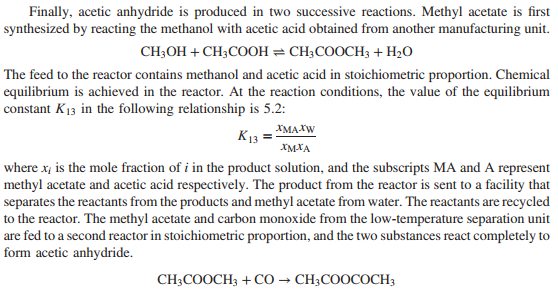

20. Before it can be fed to the gasification reactor, coal is ground to meet specifications on particle size. Since the ratio of dry coal to waterin the feed to the gasifieris to be 2kg coal /kg water, this ratio is set with the water added to the grinding and classification operations. The reactor in which gasification is to be carried out is an entrained -flow gasifier. A coal-water slurry is fed to the gasifier along with highpressure oxygen . Both feed streams may be assumed to be at 25C. The reactor is operated adiabatically at 6300kPa absolute. At these conditions , only trace quantities of hydrocarbon by products other than methane are present in the gas leaving the gasifier, and essentially all of the reactive carbon, hydrogen, and sulfur in the coal are reacted. The gas and molten ash (slag) leaving the gasifier are fed to a wash tank where the gas is bubbled through water and the slag is solidified. The gas leaving the wash tank isfed to a scrubber where it contacts a water spray. Small amounts of residual particulate matter are removed from the gas, which is also cooled from 220C to 170C. The water with which the gas iscontacted has been pumped from the wash tank, through aheat exchanger, and into the scrubber. The gas-liquid mixture formed in the scrubberis separated into saturated gas and liquid streams at 170 C and 5960kPa absolute, and the water is returned to the wash tank. Solids collected in the wash tank are allowed to settle, and are then removed along with the water condensed from the gas leaving the gasifier. The composition of the gas leaving the gasifieris given in below. \begin{tabular}{l|cccccc} \hline Component & CO & H2 & CO2 & N2 & CH4 & H2S \\ \hline mole\% & 47.95 & 37.25 & 13.85 & 0.6 & 0.1 & 0.25 \\ \hline \end{tabular} The ratio of hydrogen to carbon monoxide in the gas leaving the scrubber must be adjusted to satisfy stoichiometric requirements associated with downstream reactions. The adjustment is made by contacting aportion of the gas leaving the scrubber with acatalyst that increases therate at which the water-gas shift reaction occurs. The shift reactor consists of a fixed bed of cobalt-molybdenum catalyst pelletsandoperatesat 300C and 5615kPaabsolute. The product from the water-gas shift reactor and theunreacted portion of thesynthesis gas stream obtained from the gasifierare blended and cooled by generating steam in waste-heat boilers. Water is removed completely from the cooled gas in a molecular sieve dryer. The dry gas isthen fed to an acidgas removal system(AGRS) where all of the sulfur-containing compounds are separated and sent to a sulfur-recovery reactor, and 99.9% of the CO2 is separated and either vented or recovered for use elsewhere in the plant. The AGRS consists of two absorption columns, both of which operate at 5000kPaabsolute, and a solvent regeneration section. The gas enters the first absorption column and contacts refrigerated methyl alcohol flowing countercurrently to the gas. In the column, some CO2 and essentially all of the H2S are absorbed in the methyl alcohol. The gas leaving this column is sent to a second absorption column where most of the remaining CO2 isabsorbed in additional methylalcohol. The gasexiting the second column is at 30C and contains 0.1% of the CO2 that entered the first absorber with the crude coalgas. There are two separate operations in the regeneration section of the AGRS. In the first, the methyl alcohol from the first absorber is flashed (abruptly exposed to a lower pressure), causing a significant fraction of the absorbed gases to come out of solution. Essentially all of the absorbed CO2 and H2S are recovered in the off-gas, and the regenerated methylalcohol isrecirculated to the first absorber. The offgas from the flash tank is a mixture consisting of 15mole%H2S and 85%CO2. In the second operation, methyl alcohol from the second absorber is fed to a stripping column where it flows downward to a reboiler. Indirect steam heating (heat transferred through a heat transfer surface ) is used to vaporize a portion of the methyl alcohol, which isreturned to the column and strips the CO2 from the liquid methyl alcohol. Theregenerated liquid methylalcohol is cooled and recirculated tothe second absorber. Sulfur emissions are potential health hazards, and safe disposal of the H2S separated from the product gas is an important provision of the proposed plant. Toaccomplish this, thehydrogen sulfide is converted into elemental sulfur in a Claus process. In this process, the H2S-rich gas discharged from the flash tank is split, with one-third going to a furnace where the hydrogen sulfide is burned at 1atm with a stoichiometric amount of air to form SO2. H2S+23O2SO2+H2O composition of coal to be used as feed to the gasifier Note: The coal contains 10.5% moisture. The hydrogen and oxygen percentages given here include the masses of these elements in the coal moisture. The hot gases leave the furnace and are cooled prior to being mixed with the remainder of the H2S rich gas from the flash tank. The mixed gas is then fed to a catalytic reactor where hydrogen sulfide and SO2 react to form elemental sulfur. 2H2S+SO22H2O+3S The gases leave the reactor at 380C and are cooled to 130C by generating steam at 205kPa from saturated liquid at 205kPa. Liquid sulfur is condensed and recovered as the hot gases are cooled at atmospheric pressure. As an intermediate step in the production of acetic anhydride, carbon monoxide and carbon dioxide are reacted with hydrogen to produce methanol. 1. CO+2H2CH3OH 2. CO2+3H2CH3OH+H2O These reactions are carried out at 200C and 4925kPa absolute. The feed to the methanol reactor is formed by combining recycle from the reactor with a fresh feed stream containing 5% excess hydrogen, based on complete conversion of CO and CO2 to methanol. The composition of the gas leaving the AGRS is adjusted to meet the fresh-feed requirement on excess hydrogen. This is accomplished by splitting the gas from the second absorption column into two streams, one of which is sent to a low-temperature separation unit while the second is blended with the hydrogenrich stream exiting this unit. The separation is designed to condense 95% of the inlet carbon monoxide and to operate at 5000kPa. Reactions 1 and 2 do not go to completion, and conversions of CO and CO2 must be estimated basedon equilibrium constants available from the literature. The equilibrium constant K sfor Reaction 1 is givenhere interms of the partial pressures of reactants and products in kPaand system temperature in Kelvin2 K8=pCOpH2pM=1.390104exp[21.225+(9143.6/T)7.492lnT+4.076103T7.161108T2] where the subscripts M and Hrepresent methanol and H2, respectively . Note that Reaction 2 can be represented asthe sum of Reaction 1 and thereverse of the water-gas shiftreaction 3. H2+CO2CO+H2O The equilibrium constant for Reaction 3 is given by theequation K11=pCO2pHpCOpW=3.330exp[13.148(5639.5/T)1.077lnT5.44104T+1.125107T2+(49,170/T2)] where the subscript W represents water. The stream leaving the reactor is sent to a multiple-unit process that separates methanol and water from the noncondensables, and then separates the methanol and water. The stream of noncondensables, which consists of carbon dioxide, carbon monoxide, hydrogen, methane, and nitrogen, is sent to a multiple-unit process that removes all of the nitrogen and methane and some of the hydrogen. The gas that remains contains the quantity of hydrogen stoichiometrically required to convert all of the CO and CO2 to methanol. This stream is recycled to join the fresh feed to the reactor. Finally, acetic anhydride is produced in two successive reactions. Methyl acetate is first synthesized by reacting the methanol with acetic acid obtained from another manufacturing unit. CH3OH+CH3COOHCH3COOCH3+H2O The feed to the reactor contains methanol and acetic acid in stoichiometric proportion. Chemical equilibrium is achieved in the reactor. At the reaction conditions, the value of the equilibrium constant K13 in the following relationship is 5.2: K13=xMxAxMAxW where xi is the mole fraction of i in the product solution, and the subscripts MA and A represent methyl acetate and acetic acid respectively. The product from the reactor is sent to a facility that separates the reactants from the products and methyl acetate from water. The reactants are recycled to the reactor. The methyl acetate and carbon monoxide from the low-temperature separation unit are fed to a second reactor in stoichiometric proportion, and the two substances react completely to form acetic anhydride. CH3COOCH3+COCH3COOCOCH3 20-1 Assume there are no side reactions in the synthesis of methanol, methyl acetate, and acetic anhydride, and calculate the molar flow rates of reactants (kmol/h) in the fresh feeds to each of the reactors. Where fresh feed must be supplied from a source external to the process, calculate the feed rates of these materials in kg/h. Construct a detailed flow chart of the process, showing known compositions, flow rates, pressures, and temperatures. 20-2 Estimate the amount of oxygen fed to the gasification reactor in kmol and standard cubic meters per kg of coal fed to the process. 20-3 If all of the sulfur in the coal reacts to form H2S, determine the air requirements for the Claus plant in kmol and standard m3 when 1kg of coal is fed to the process. 20-4 Estimate the mole fraction of water in the gas exiting the scrubber. What is the rate (kg/kg coal) at which water exits the scrubber in the product gas? 20-5 Determine the coal feed rate (kg/h), and calculate the rates at which oxygen is fed to the gasifier (kmol/h) and air is fed to the Claus plant (kmol/h). 20-6 Based on the equilibrium constant given for Reaction CS5.13, calculate the composition of the product stream leaving the methyl acetate synthesis reactor and the single-pass conversion of methanol to methyl acetate. Determine the recycle rates of methanol and acetic acid. (Hint: For the single-pass conversion, the definition of conversion XA is the number of moles of A that have reacted per mole of A fed to the system) XA=MolesofAfedMolesofAreacted 20-7 Assume that the temperature of the gases and slag leaving the gasifier is 1100C. Estimate the total rate at which heat must be removed from the wash tank-scrubber system. (Note: The actual temperature of such streams in commercial entrained-flow gasifiers is closer to 1350C.) The following properties of ash and slag may be used: For ash, Cp(kJ/kgC)=0.604+0.586103T(K),H^m=710kJ/kg; for slag, Cp(kJ/kgC)=0.767, +0.744103T(K). 20-8 At what rate (kW) is heat transferred to or from (state which) the methanol synthesis reactor to maintain its operation at 200C ? You may assume that the recycle stream is at 40C, and the fresh feed is at 20C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


