Question
The human body contains many elements from the periodic table. It is mostly composed of oxygen and carbon, but trace elements also have a
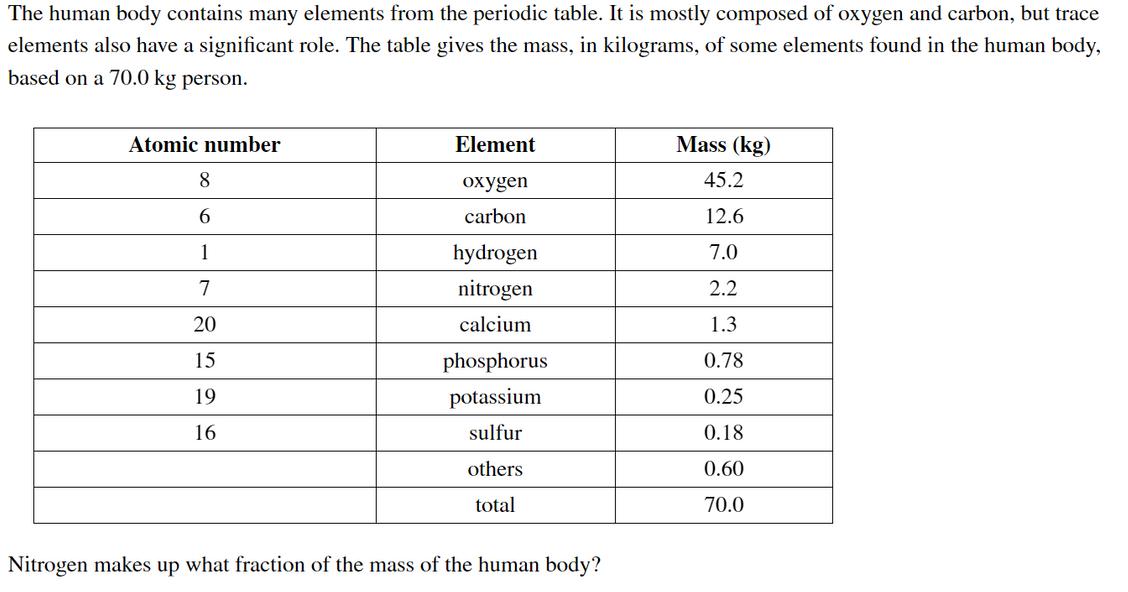
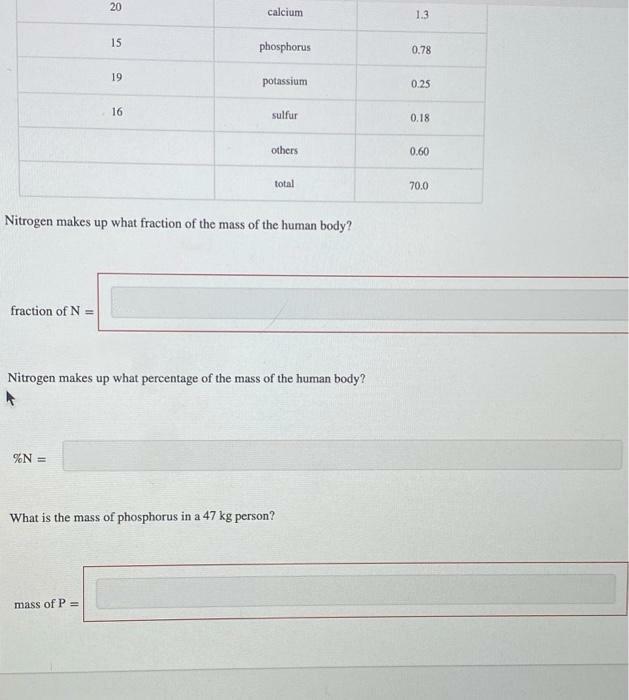
The human body contains many elements from the periodic table. It is mostly composed of oxygen and carbon, but trace elements also have a significant role. The table gives the mass, in kilograms, of some elements found in the human body, based on a 70.0 kg person. Atomic number Element Mass (kg) 8 oxygen 45.2 6. carbon 12.6 1 hydrogen 7.0 7 nitrogen 2.2 20 calcium 1.3 15 phosphorus 0.78 19 potassium 0.25 16 sulfur 0.18 others 0.60 total 70.0 Nitrogen makes up what fraction of the mass of the human body? 20 calcium 1.3 15 phosphorus 0.78 19 potassium 0.25 16 sulfur 0.18 others 0.60 total 70.0 Nitrogen makes up what fraction of the mass of the human body? fraction of N = Nitrogen makes up what percentage of the mass of the human body? %N = What is the mass of phosphorus in a 47 kg person? mass of P =
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Conceptual Physics
Authors: Paul G. Hewitt
11th edition
321568095, 9780-032166256, 321662563, 978-0321568090
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



