Answered step by step
Verified Expert Solution
Question
1 Approved Answer
[20pts] 2. Liquid a and b are known to form a binary mixture defined by the following excess Gibbs equation: GE=6000xaxb(10.0005T)[J/mol] where T is in
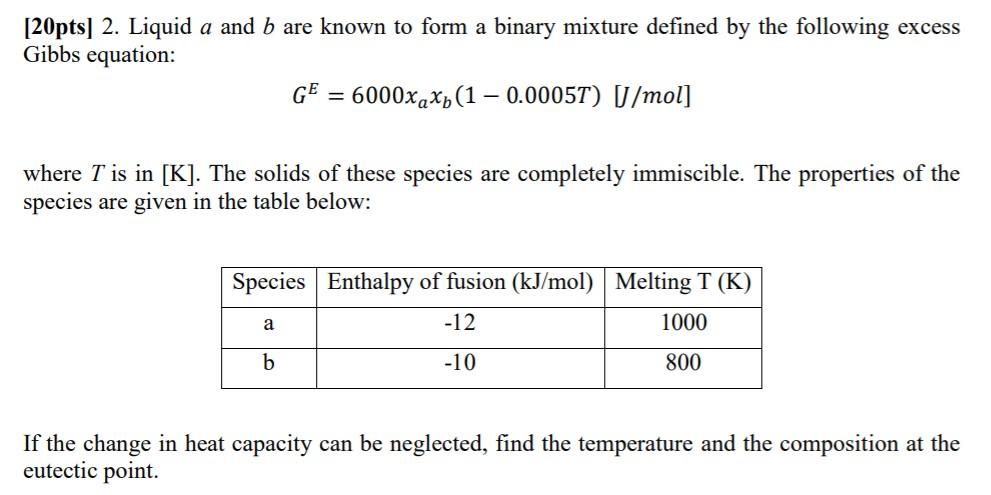
[20pts] 2. Liquid a and b are known to form a binary mixture defined by the following excess Gibbs equation: GE=6000xaxb(10.0005T)[J/mol] where T is in [K]. The solids of these species are completely immiscible. The properties of the species are given in the table below: If the change in heat capacity can be neglected, find the temperature and the composition at the eutectic point. [20pts] 2. Liquid a and b are known to form a binary mixture defined by the following excess Gibbs equation: GE=6000xaxb(10.0005T)[J/mol] where T is in [K]. The solids of these species are completely immiscible. The properties of the species are given in the table below: If the change in heat capacity can be neglected, find the temperature and the composition at the eutectic point
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


