Answered step by step
Verified Expert Solution
Question
1 Approved Answer
21, 23 and 25 question number 21 23 and 25 a. 21. A reaction is 50 % complete in 30.0 min. How long after its
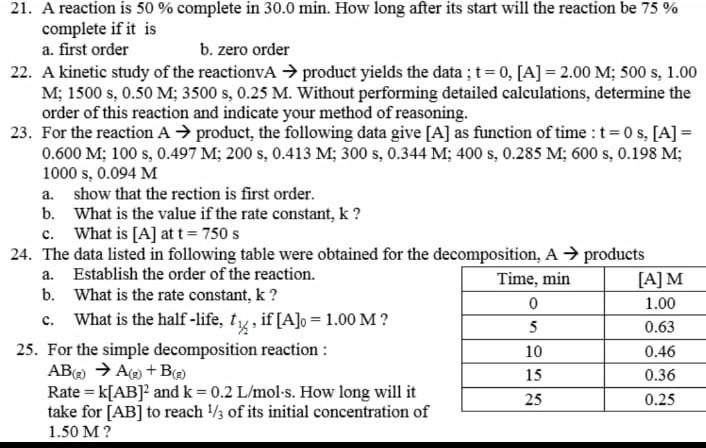
21, 23 and 25
question number 21 23 and 25
a. 21. A reaction is 50 % complete in 30.0 min. How long after its start will the reaction be 75 % complete if it is a. first order b. zero order 22. A kinetic study of the reactionvA product yields the data ; t=0, [A] = 2.00 M; 500 s, 1.00 M; 1500 s, 0.50 M; 3500 s, 0.25 M. Without performing detailed calculations, determine the order of this reaction and indicate your method of reasoning. 23. For the reaction A product, the following data give [A] as function of time : t=0 s, [A] = 0.600 M; 100 s, 0.497 M; 200 s, 0.413 M; 300 s, 0.344 M; 400 s, 0.285 M; 600 s, 0.198 M; 1000 s, 0.094 M show that the rection is first order. b. What is the value if the rate constant, k? c. What is [A] at t= 750 s 24. The data listed in following table were obtained for the decomposition, A products a. Establish the order of the reaction. Time, min [A]M b. What is the rate constant, k? 0 1.00 c. What is the half-life, ty, if [A]o = 1.00 M? 5 0.63 25. For the simple decomposition reaction : 10 0.46 AB) A(+B 15 0.36 Rate = k[AB] and k=0.2 L/mol-s. How long will it 25 0.25 take for [AB] to reach 1/3 of its initial concentration of 1.50 MStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


