Answered step by step
Verified Expert Solution
Question
1 Approved Answer
21.For the reaction: X2+ Y = 2XY Activation energies for forward and backward reactions are 90 kJ mol-1 and 100 kJ mol. On addition
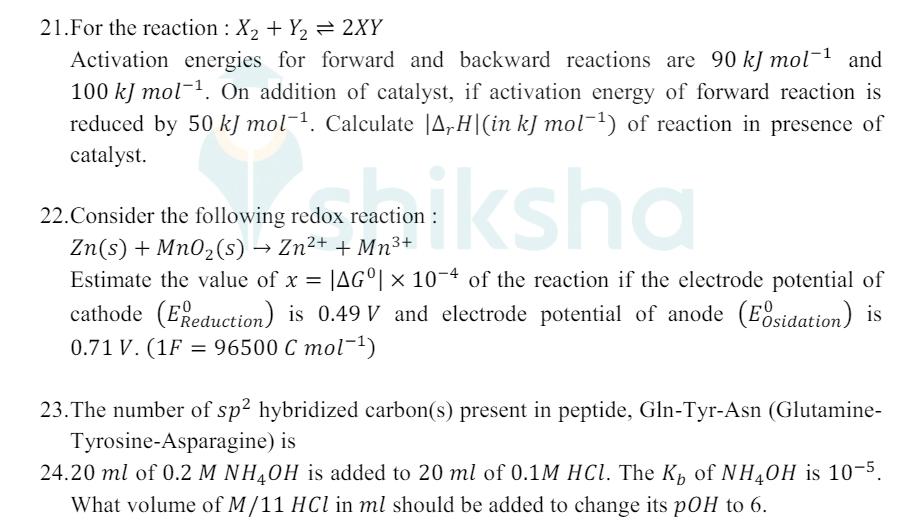
21.For the reaction: X2+ Y = 2XY Activation energies for forward and backward reactions are 90 kJ mol-1 and 100 kJ mol. On addition of catalyst, if activation energy of forward reaction is reduced by 50 kJ mol-1. Calculate |A,H|(in kJ mol) of reaction in presence of catalyst. de reaction: ksha 22. Consider the following redox reactio Zn(s) + MnO2(s) Zn+ + Mn+ Estimate the value of x = |AG| 10-4 of the reaction if the electrode potential of cathode (E Reduction) is 0.49 V and electrode potential of anode (Eosidation) is 0.71 V. (1F = 96500 C mol-) 23. The number of sp hybridized carbon(s) present in peptide, Gln-Tyr-Asn (Glutamine- Tyrosine-Asparagine) is 24.20 ml of 0.2 M NH4OH is added to 20 ml of 0.1M HCl. The K of NH4OH is 10-5. What volume of M/11 HCl in ml should be added to change its pOH to 6.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


