Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(23pts) Part 2: Making Verdigris, Copper Acetate (0.5pts) Mass CuSO4 5H0 (g) (1pts) Moles CuSO4 5H0 (mol) (2pts) What is the colour of copper(11)
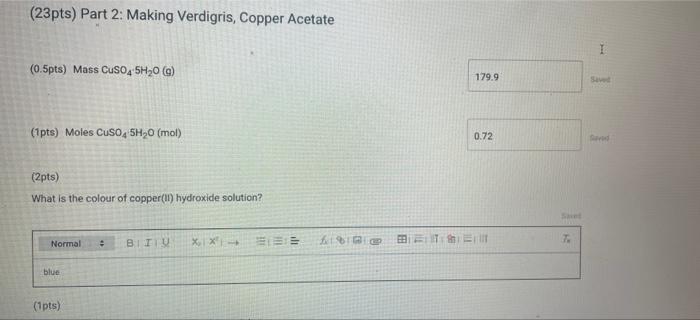
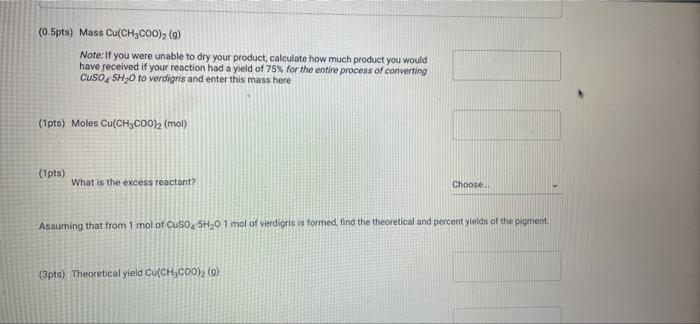
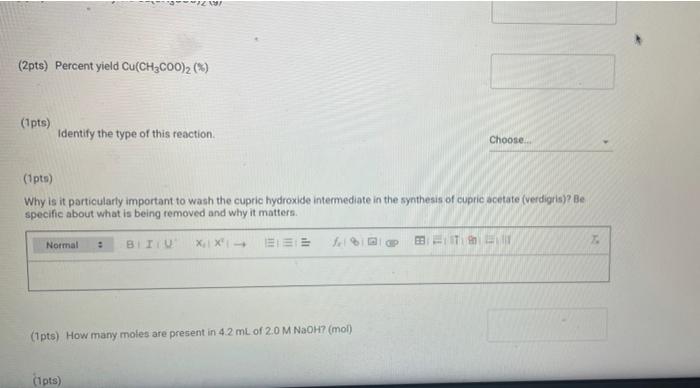
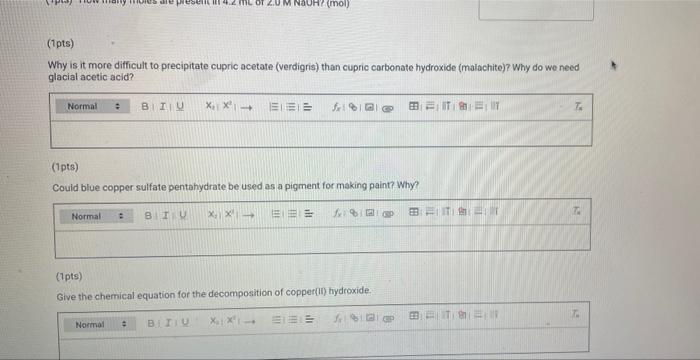
(23pts) Part 2: Making Verdigris, Copper Acetate (0.5pts) Mass CuSO4 5H0 (g) (1pts) Moles CuSO4 5H0 (mol) (2pts) What is the colour of copper(11) hydroxide solution? Normal blue (1pts) # BITU E 6853 TE 179.9 0.72 Savet T I Saved Seved (0.5pts) Mass Cu(CHCOO)2 (9) Note: If you were unable to dry your product, calculate how much product you would have received if your reaction had a yield of 75% for the entire process of converting CuSO4 SHO to verdigris and enter this mass here (1pts) Moles Cu(CHC00)2 (mol) (1pts) What is the excess reactant? 60 (3pts) Theoretical yield Cu(CHCO0)2 (0) Choose... Assuming that from 1 mol of CuSO, SH0 1 mol of verdigris is formed, find the theoretical and percent yields of the pigment. (2pts) Percent yield Cu(CH3COO)2 (%) (1pts) MINI Identify the type of this reaction. Normal : (1pts) Why is it particularly important to wash the cupric hydroxide intermediate in the synthesis of cupric acetate (verdigris)? Be specific about what is being removed and why it matters. BIU X X (1pts) How many moles are present in 4.2 mL of 2.0 M NaOH? (mol) (1pts) Choose.... BET es are preselil 4.2 mL of 2.0 M NaOH? (mol) (1pts) Why is it more difficult to precipitate cupric acetate (verdigris) than cupric carbonate hydroxide (malachite)? Why do we need glacial acetic acid? Normal BIU X X4 Normal (1pts) Could blue copper sulfate pentahydrate be used as a pigment for making paint? Why? EEE : BIU X, X Normal : BIZU EEE 18 (1pts) Give the chemical equation for the decomposition of copper(1) hydroxide. X X BEITSE BES f BT T Ta 72
Step by Step Solution
★★★★★
3.41 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
1Moles of CuSO45H2O mol 072 mols 2 CopperII hydroxide solution is typically bluegreen We need the ba...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


