Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2.4 mscf/d of the gas is compressed adiabatically and reversibly from 100 psi and 110F to 1000 psi (i) What is the final temperature

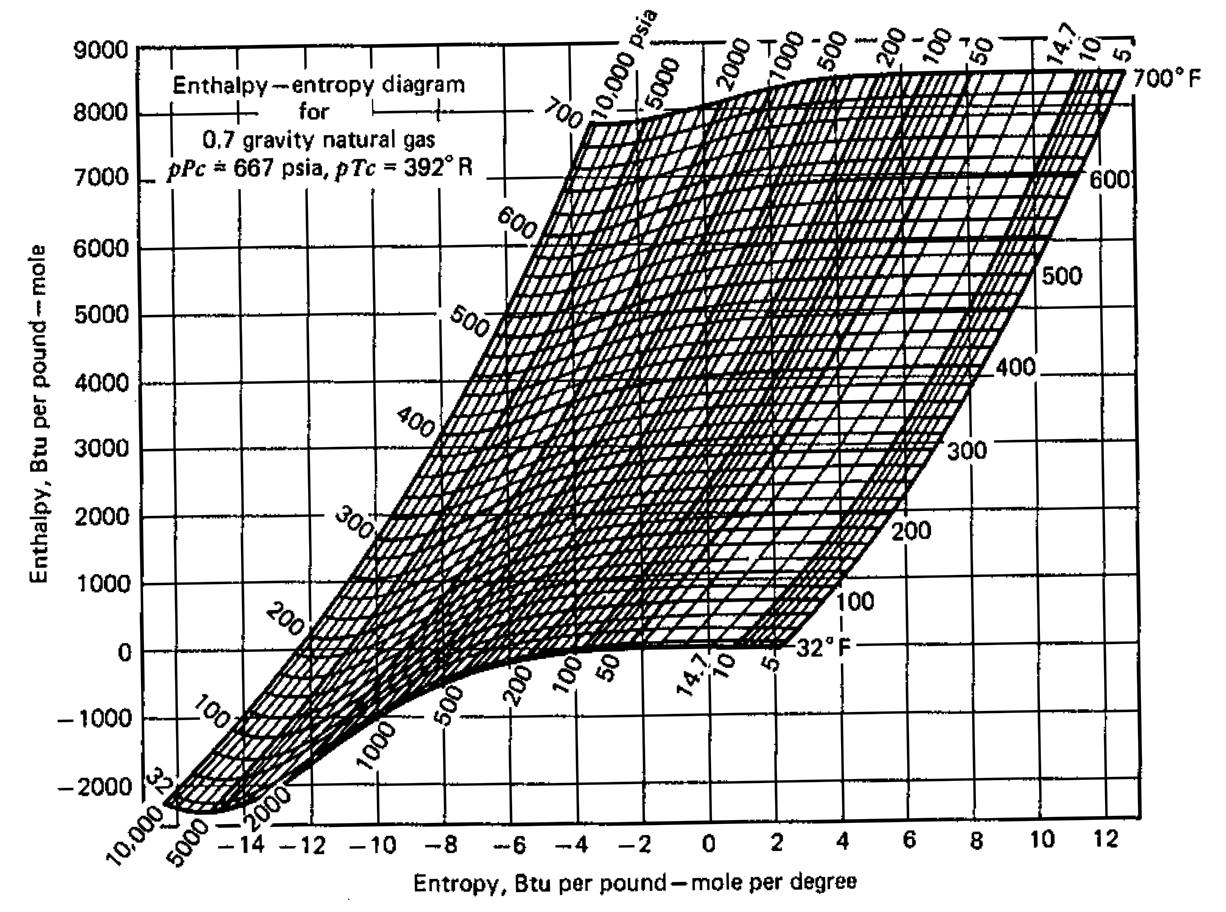
2.4 mscf/d of the gas is compressed adiabatically and reversibly from 100 psi and 110F to 1000 psi (i) What is the final temperature of this gas (ii) What is the energy requirement of this process (ii) What is the energy (in horsepower) requirement of the compressor 1 Horsepower = 2545 BTU/hr Use information provided at the end. Please explain how you calculate your answers. 700 F 9000 Enthalpy-entropy diagram for 700 8000 600. 0,7 gravity natural gas pPc * 667 psia, pTc = 392 R 7000 600 500 6000 500 400 5000 4000 400 300 3000 200 2000 100 1000 200 32 F 100 - 1000 10 12 -2000 4 8 2 -8 -6 -4 -2 - 14 -12 -10 Entropy, Btu per pound-mole per degree 200 00s 00 w00 00s 000 000, 0009 10,000 psia Enthalpy, Btu per pound -mole
Step by Step Solution
★★★★★
3.54 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Given Initial pressure R10o psi 2 1000 psi Final ressure I psi 689476 Pa Also Init...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


