Question
25.4g of NaOH are dissolved in water within an insulated calorimeter. The heat capacity of the resulting solution is 4.18 x 10^3J/C. The temperature
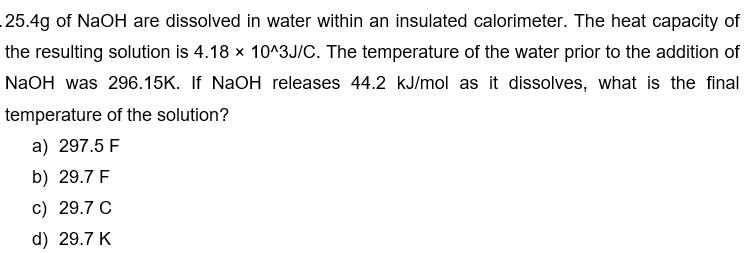
25.4g of NaOH are dissolved in water within an insulated calorimeter. The heat capacity of the resulting solution is 4.18 x 10^3J/C. The temperature of the water prior to the addition of NaOH was 296.15K. If NaOH releases 44.2 kJ/mol as it dissolves, what is the final temperature of the solution? a) 297.5 F b) 29.7 F c) 29.7 C d) 29.7 K
Step by Step Solution
3.50 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
ans Heat capacity d detihed as amount of heat reeure tes rafop the demperatune bo one cenilt C of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Joseph M. Hornback
2nd Edition
9781133384847, 9780199270293, 534389511, 1133384846, 978-0534389512
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



