Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2.5.A Determine which of the following proposed fundamental relations satisfy the postulates of thermodynamics. From textbook: de Pablo and Schiebers Molecular Engineering Thermodynamics. 1. S=s0N+AU1/4V1/2N1/4,
2.5.A Determine which of the following proposed fundamental relations satisfy the postulates of thermodynamics.
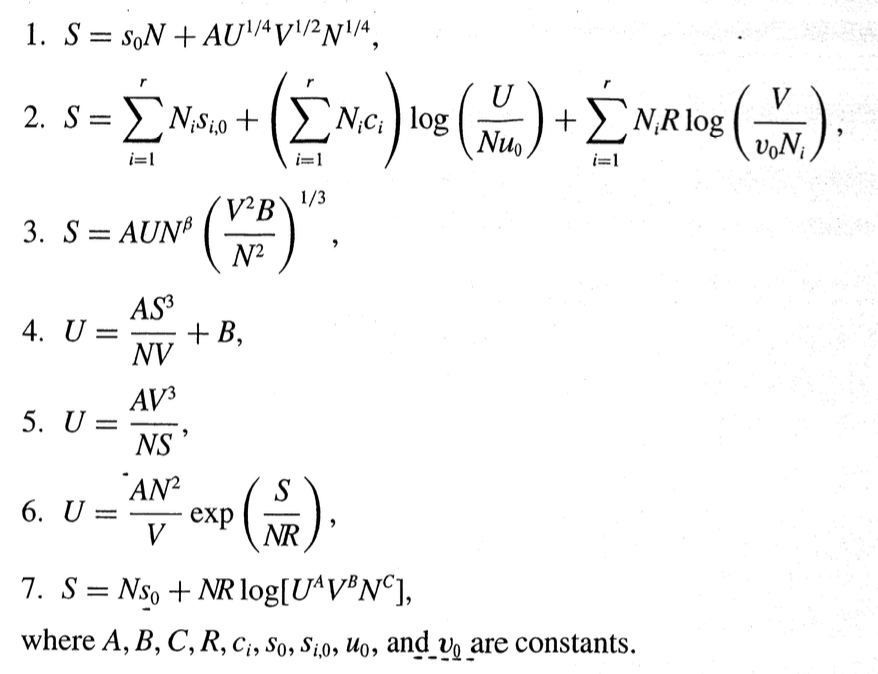
From textbook: de Pablo and Schiebers Molecular Engineering Thermodynamics.
1. S=s0N+AU1/4V1/2N1/4, 2. S=i=1rNisi,0+(i=1rNici)log(Nu0U)+i=1rNiRlog(v0NiV), 3. S=AUN(N2V2B)1/3, 4. U=NVAS3+B, 5. U=NSAV3 6. U=VAN2exp(NRS), 7. S=Ns0+NRlog[UAVBNC], where A,B,C,R,ci,s0,si,0,u0, and v0 are constantsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


