Question
A liquid-liquid extraction process conducted in Electrochemical Materials Laboratory involved the extraction of nickel from the aqueous phase into an organic phase. A typical
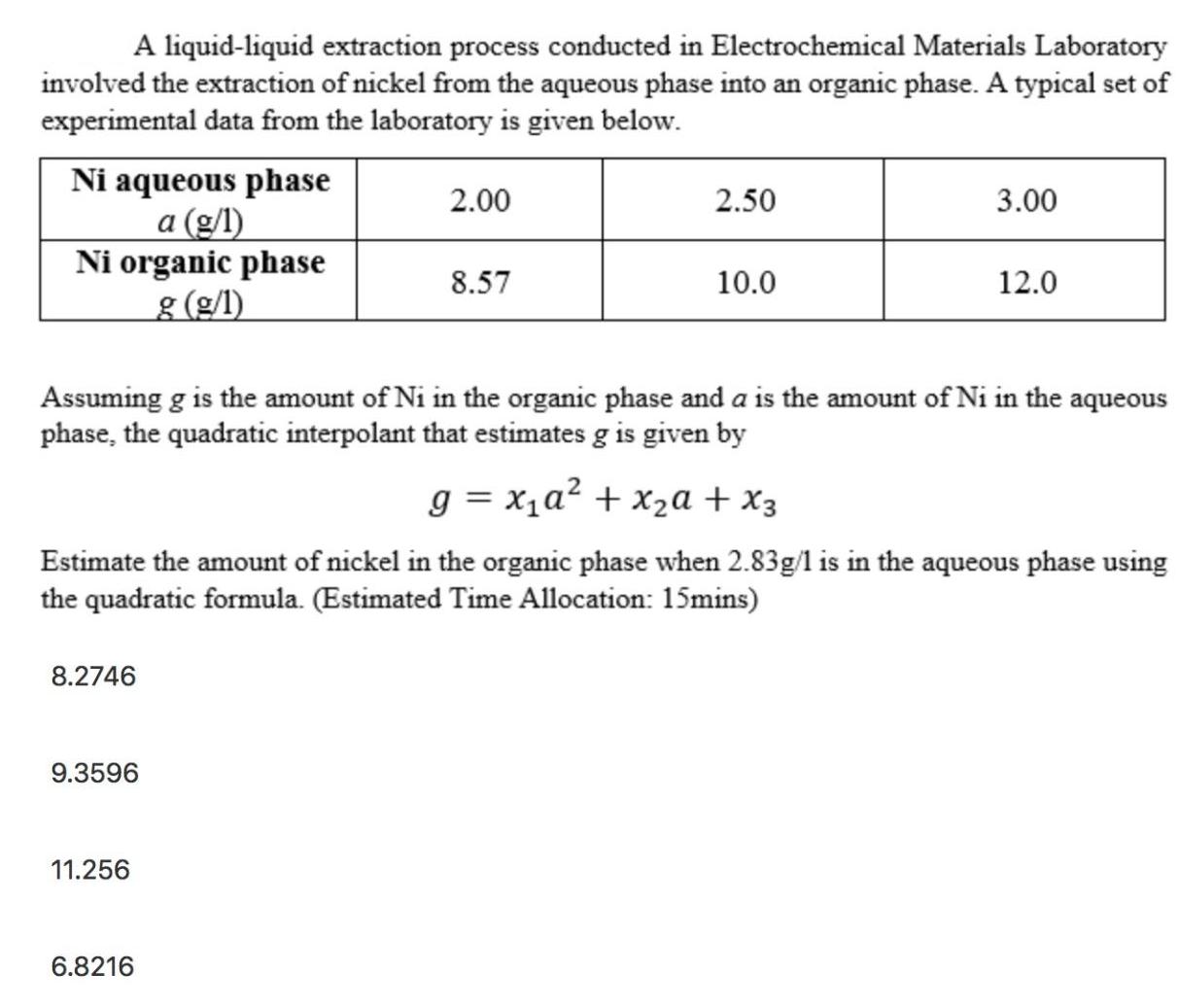
A liquid-liquid extraction process conducted in Electrochemical Materials Laboratory involved the extraction of nickel from the aqueous phase into an organic phase. A typical set of experimental data from the laboratory is given below. Ni aqueous phase 2.00 2.50 3.00 a (g/l) Ni organic phase 8.57 10.0 12.0 g (g/l) Assuming g is the amount of Ni in the organic phase and a is the amount of Ni in the aqueous phase, the quadratic interpolant that estimates g is given by g = xa + x + x3 Estimate the amount of nickel in the organic phase when 2.83g/1 is in the aqueous phase using the quadratic formula. (Estimated Time Allocation: 15mins) 8.2746 9.3596 11.256 6.8216
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Separation process principles
Authors: J. D. Seader
2nd Edition
471464805, 978-0471464808
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



