Answered step by step
Verified Expert Solution
Question
1 Approved Answer
26 The number of protons, neutrons, electrons in some particles are shown in the table below Particle Protons Neutrons electrons P 1 1 2
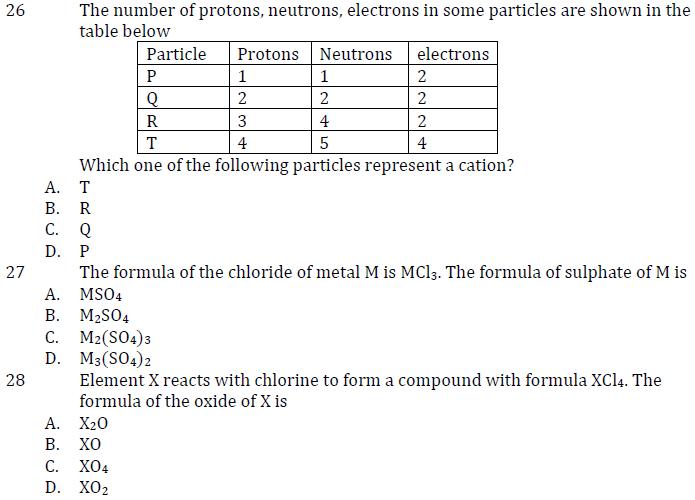
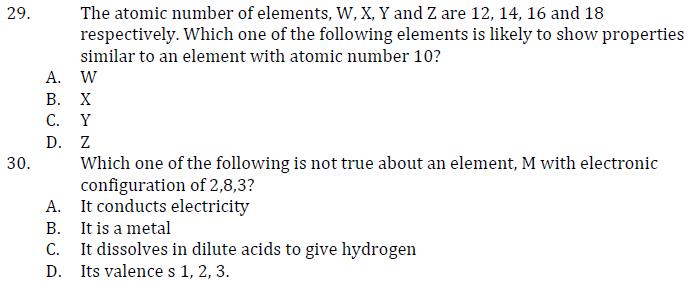
26 The number of protons, neutrons, electrons in some particles are shown in the table below Particle Protons Neutrons electrons P 1 1 2 Q 2 2 2 R 3 4 2 T 4 5 4 Which one of the following particles represent a cation? A. T B. R C. Q P 27 D. 28 A. The formula of the chloride of metal M is MC13. The formula of sulphate of M is MSO4 B. MSO4 C. M2(SO4)3 D. M3(SO4)2 Element X reacts with chlorine to form a compound with formula XC14. The formula of the oxide of X is A. X20 B. XO C. XO4 D. XO2 29. The atomic number of elements, W, X, Y and Z are 12, 14, 16 and 18 respectively. Which one of the following elements is likely to show properties similar to an element with atomic number 10? Z A. W B. X C. Y D. 30. A. Which one of the following is not true about an element, M with electronic configuration of 2,8,3? It conducts electricity B. It is a metal C. It dissolves in dilute acids to give hydrogen D. Its valence s 1, 2, 3.
Step by Step Solution
★★★★★
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
26 A cation is a positively charged ion which means it has lost electrons Looking at the given parti...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


