Answered step by step
Verified Expert Solution
Question
1 Approved Answer
27.On heating boric acid, oxide 'A' is formed. On reacting 'A' with NaOH,'B' is formed. How many moles of A would be required to
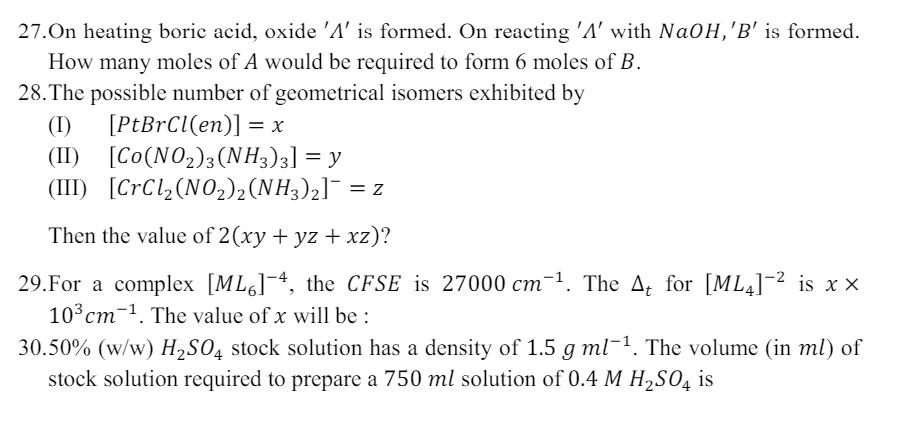
27.On heating boric acid, oxide 'A' is formed. On reacting 'A' with NaOH,'B' is formed. How many moles of A would be required to form 6 moles of B. 28. The possible number of geometrical isomers exhibited by (I) (II) [PtBrCl(en)] = x [Co(NO2)3(NH3)3] = y (III) [CrCl(NO2)2(NH3)2] = z Then the value of 2(xy + yz + xz)? 29. For a complex [ML6]-4, the CFSE is 27000 cm. The At for [ML4] is xx 103cm . The value of x will be : 30.50% (w/w) H2SO4 stock solution has a density of 1.5 g ml. The volume (in ml) of stock solution required to prepare a 750 ml solution of 0.4 M H2SO4 is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


