Answered step by step
Verified Expert Solution
Question
1 Approved Answer
28. A steel part with surface area of 125 cm is to be chrome coated through an electroplating process using chromium acid sulphate as
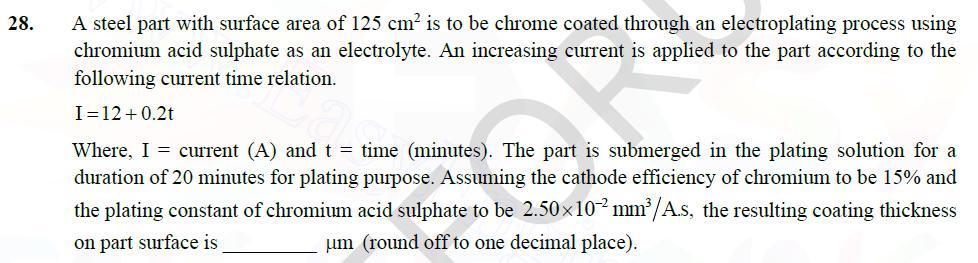
28. A steel part with surface area of 125 cm is to be chrome coated through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation. I=12+0.2t Where, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50102 mm/A.s, the resulting coating thickness on part surface is um (round off to one decimal place).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


