Question
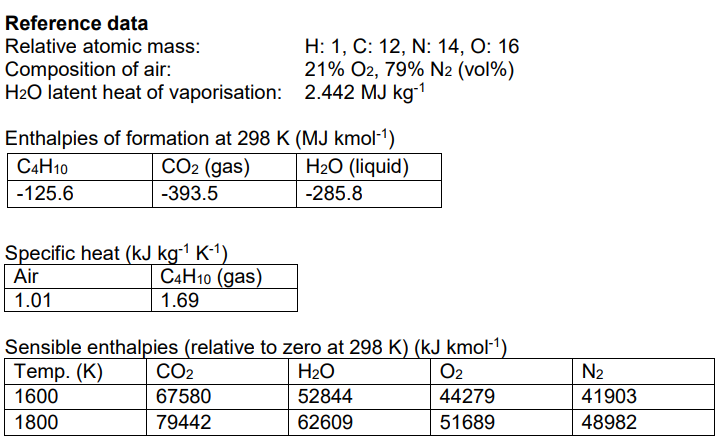
2a)Using enthalpies of formation and other data, from the reference data provided below, calculate the net and gross calorific values for butane (C4H10) in units
2a)Using enthalpies of formation and other data, from the reference data provided below, calculate the net and gross calorific values for butane (C4H10) in units of MJ kmol-1 .
2b)The following data is obtained for a gas burner: Fuel: Butane (C4H10) Oxidant: Air Mixture: 3% fuel, 97% air (by volume) Fuel temperature: 15C Oxidant temperature: 160C Heat loss in combustion: 930 MJ kmol-1 (of fuel) Assume dissociation of the combustion products does not occur. Using your solution to part (a) and the reference data provided below, calculate:
The excess air in the fuel-oxidant mixture.
(ii) The oxygen content of the exhaust (vol%, dry).
(iii) The flame temperature.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


