Answered step by step
Verified Expert Solution
Question
1 Approved Answer
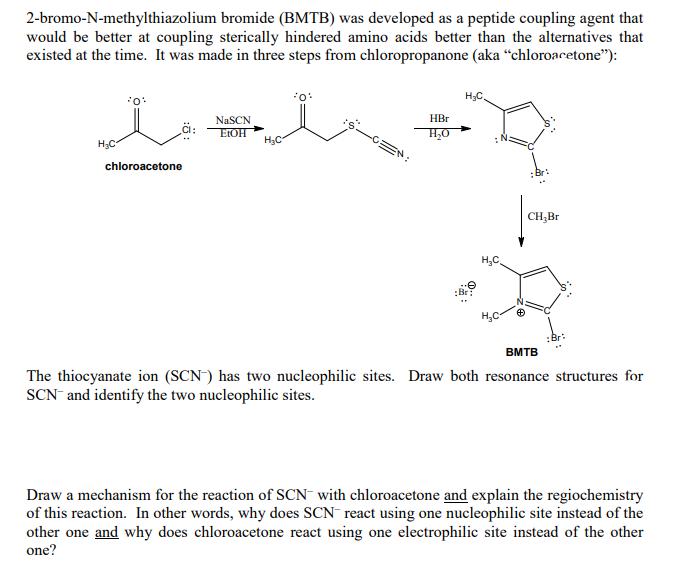
2-bromo-N-methylthiazolium bromide (BMTB) was developed as a peptide coupling agent that would be better at coupling sterically hindered amino acids better than the alternatives
![]()
2-bromo-N-methylthiazolium bromide (BMTB) was developed as a peptide coupling agent that would be better at coupling sterically hindered amino acids better than the alternatives that existed at the time. It was made in three steps from chloropropanone (aka "chloroacetone"): HC *0* CI: chloroacetone NaSCN EtOH HC *0* HBr HO HC :Br: HC CHBr ;Br: BMTB The thiocyanate ion (SCN) has two nucleophilic sites. Draw both resonance structures for SCN and identify the two nucleophilic sites. Draw a mechanism for the reaction of SCN with chloroacetone and explain the regiochemistry of this reaction. In other words, why does SCN react using one nucleophilic site instead of the other one and why does chloroacetone react using one electrophilic site instead of the other one? (c) Propose a reasonable mechanism for the second step in the synthesis of BMTB. (shown below)
Step by Step Solution
★★★★★
3.42 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


