Question
+ 2H;Oo pE =+20.8 7. (16) Consider the reaction: MnO;(s) + 4H + 2e-Mn Assume the [Mn)-0,0001 mol/L, Represent the boundary between the two
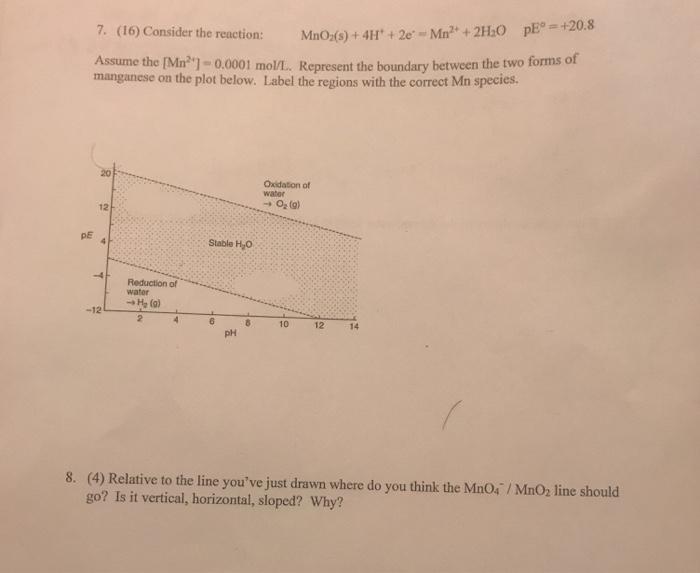
+ 2H;Oo pE =+20.8 7. (16) Consider the reaction: MnO;(s) + 4H + 2e-Mn Assume the [Mn")-0,0001 mol/L, Represent the boundary between the two forms of manganese on the plot below. Label the regions with the correct Mn species. Oxidation of water 12 DE Stable H0 Reduction of water -12 10 12 14 pH 8. (4) Relative to the line you've just drawn where do you think the MnO4 / MnO2 line should go? Is it vertical, horizontal, sloped? Why?
Step by Step Solution
3.42 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essentials of Materials Science and Engineering
Authors: Donald R. Askeland, Wendelin J. Wright
3rd edition
978-1111576868, 1111576866, 978-1285677620, 1285677625, 978-1111576851
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



