Question
2N 10 N V= 3 liters 2 N 2N V=5 liters 2. The above cylinders contain the same number of atoms of the same
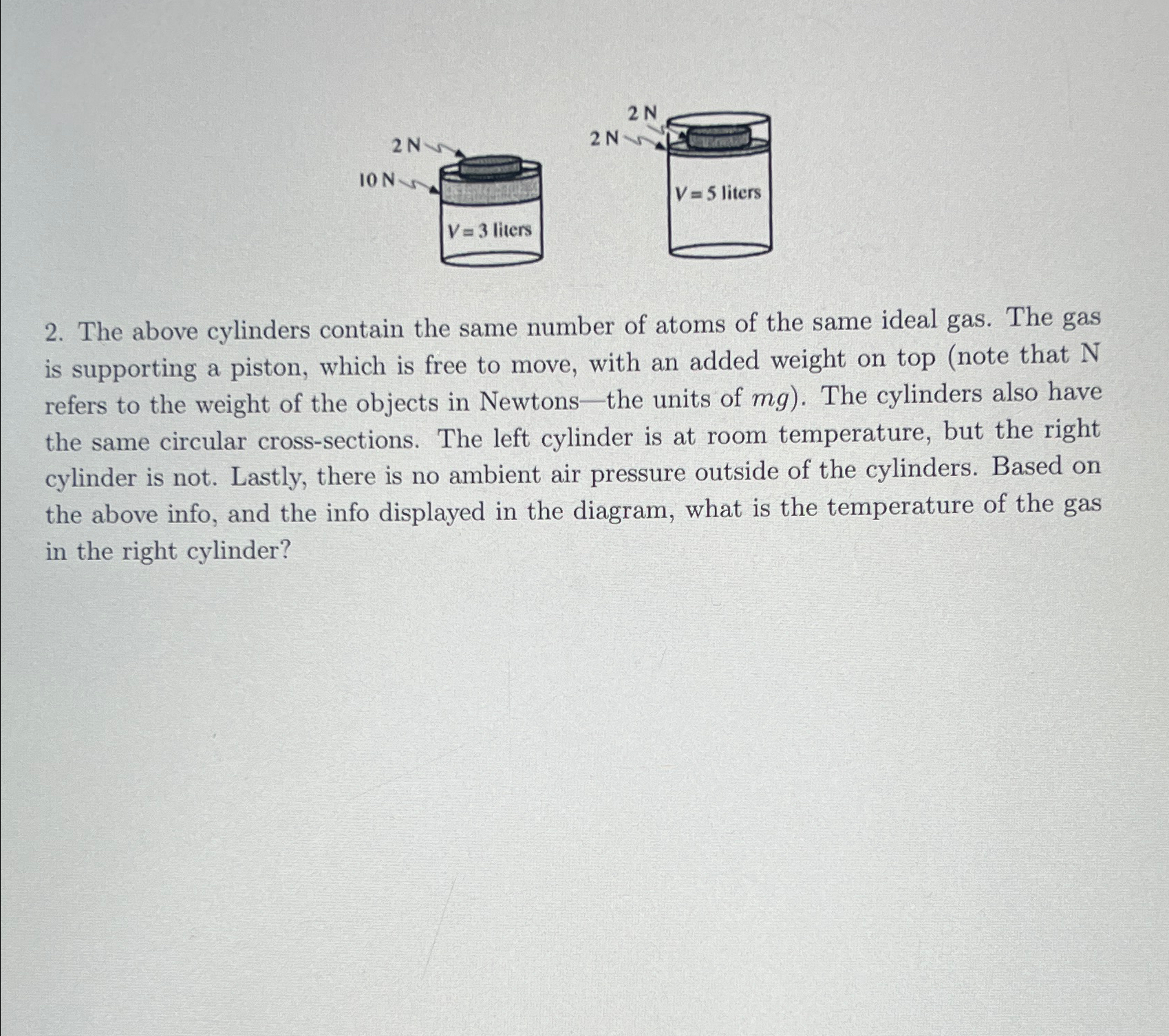
2N 10 N V= 3 liters 2 N 2N V=5 liters 2. The above cylinders contain the same number of atoms of the same ideal gas. The gas is supporting a piston, which is free to move, with an added weight on top (note that N refers to the weight of the objects in Newtons-the units of mg). The cylinders also have the same circular cross-sections. The left cylinder is at room temperature, but the right cylinder is not. Lastly, there is no ambient air pressure outside of the cylinders. Based on the above info, and the info displayed in the diagram, what is the temperature of the gas in the right cylinder?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



