Answered step by step
Verified Expert Solution
Question
1 Approved Answer
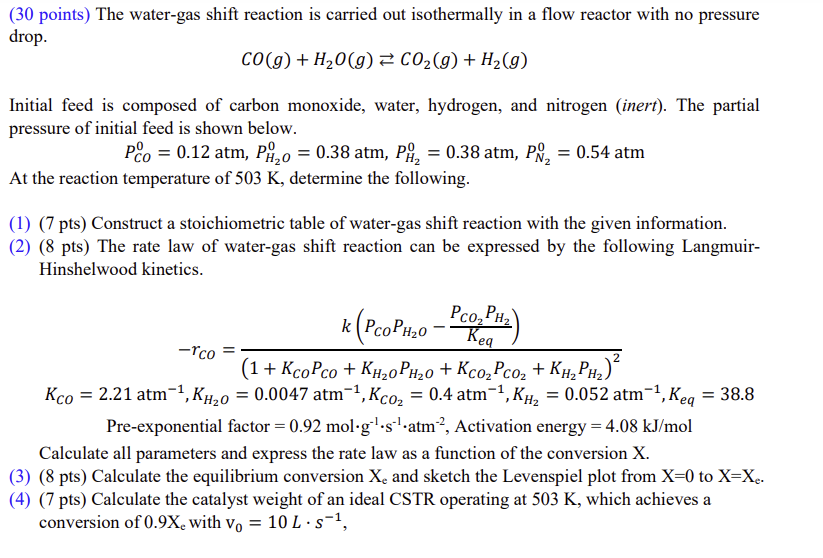
( 3 0 points ) The water - gas shift reaction is carried out isothermally in a flow reactor with no pressure drop. C O
points The watergas shift reaction is carried out isothermally in a flow reactor with no pressure
drop.
Initial feed is composed of carbon monoxide, water, hydrogen, and nitrogen inert The partial
pressure of initial feed is shown below.
atm,atm,atm,atm
At the reaction temperature of determine the following.
pts Construct a stoichiometric table of watergas shift reaction with the given information.
pts The rate law of watergas shift reaction can be expressed by the following Langmuir
Hinshelwood kinetics.
Preexponential factor mol Activation energy
Calculate all parameters and express the rate law as a function of the conversion
pts Calculate the equilibrium conversion and sketch the Levenspiel plot from to
pts Calculate the catalyst weight of an ideal CSTR operating at which achieves a
conversion of with
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


