Answered step by step
Verified Expert Solution
Question
1 Approved Answer
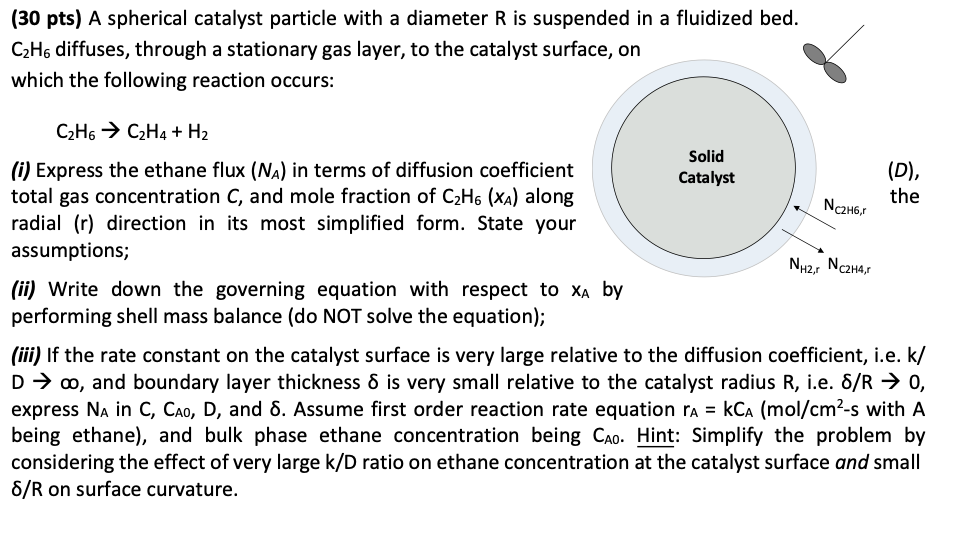
( 3 0 pts ) A spherical catalyst particle with a diameter R is suspended in a fluidized bed. C 2 H 6 diffuses, through
pts A spherical catalyst particle with a diameter is suspended in a fluidized bed.
diffuses, through a stationary gas layer, to the catalyst surfac
which the following reaction occurs:
i Express the ethane flux in terms of diffusion coefficient
total gas concentration and mole fraction of along
radial direction in its most simplified form. State your
assumptions;
ii Write down the governing equation with respect to
performing shell mass balance do NOT solve the equation;
iii If the rate constant on the catalyst surface is very large relative to the diffusion coefficient, ie
and boundary layer thickness is very small relative to the catalyst radius ie
express in and Assume first order reaction rate equation with
being ethane and bulk phase ethane concentration being Hint: Simplify the problem by
considering the effect of very large ratio on ethane concentration at the catalyst surface and small
on surface curvature.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


