Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3 . 1 0 . When an insoluble bubble rises in a deep pool of liquid, its volume increases according to the ideal gas
When an insoluble bubble rises in a deep pool of liquid, its volume increases according to the ideal gas law. However, when a soluble bubble rises from deep submersion, there is a competing action of dissolution that tends to reduce size. Under practical conditions, it has been proved Rice that the mass transfer coefficient for spherical particles or bubbles in free fall or freerise is substantially constant. Thus, for sparingly soluble bubbles released from rest, the following material balance is applicable:
where is the ideal molar density of gas, is molar solubility of gas in liquid, and is the changing bubble radius. The pressure at a distance from the top liquid surface is and the rise velocity is assumed to be quasisteady and follows the intermediate law according to Rice and Littlefield to give a linear relation between speed and size
:
note for rising bubble.
where is gravitational acceleration, and is liquid kinematic viscosity.
a Show that a change of variables allows the material balance to be written as
null
where
since
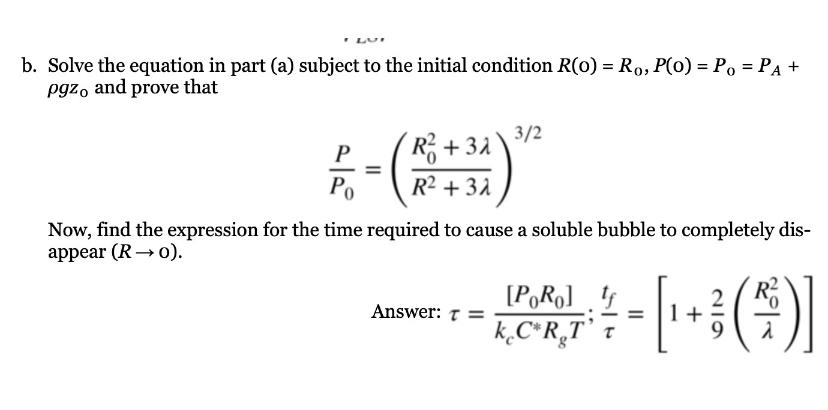
b Solve the equation in part a subject to the initial condition and prove that
Now, find the expression for the time required to cause a soluble bubble to completely disappear
Answer: ;
Only want b time expressions Please show how to get them
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


