Answered step by step
Verified Expert Solution
Question
1 Approved Answer
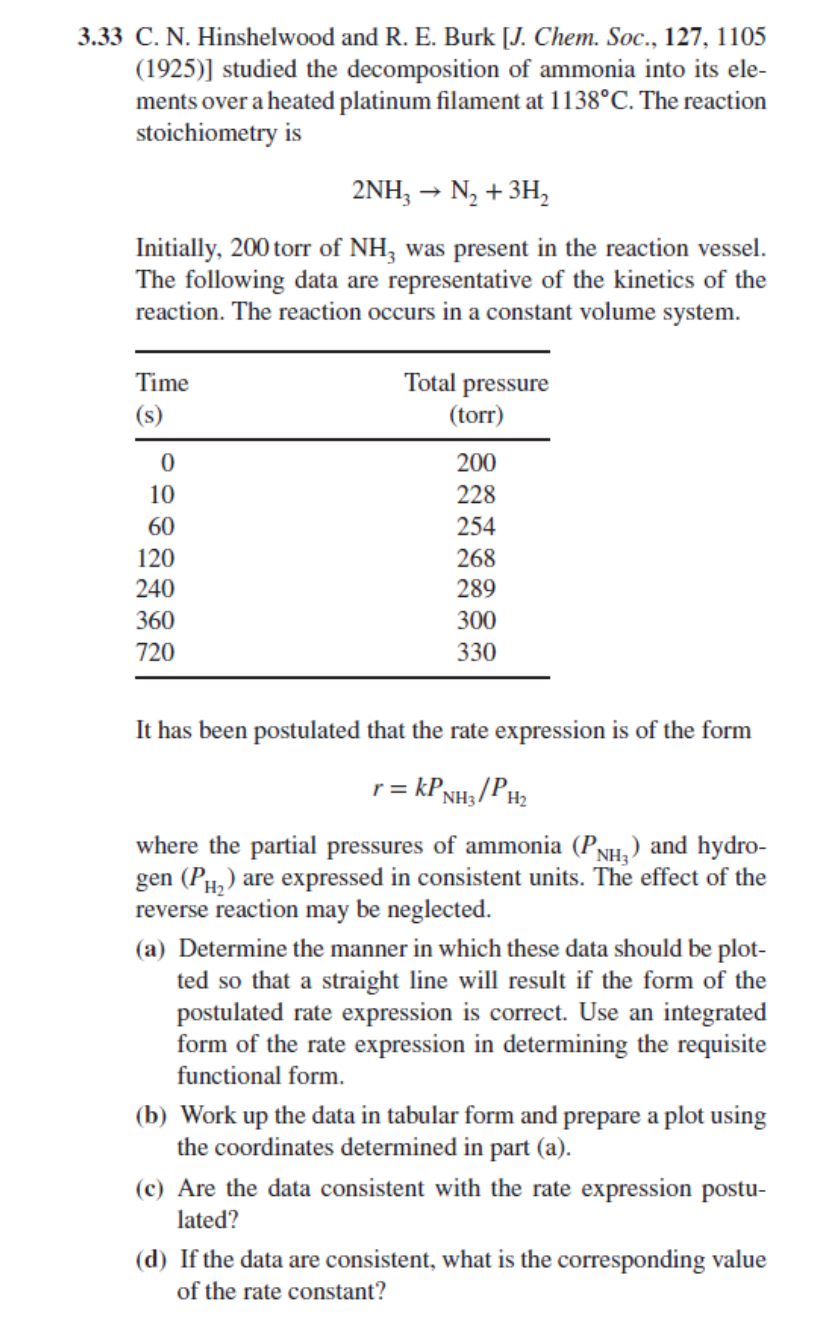
3 . 3 3 C . N . Hinshelwood and R . E . Burk [ J . Chem. Soc., 1 2 7 , 1
C N Hinshelwood and R E Burk J Chem. Soc.,
studied the decomposition of ammonia into its ele
ments over a heated platinum filament at The reaction
stoichiometry is
Initially, torr of was present in the reaction vessel.
The following data are representative of the kinetics of the
reaction. The reaction occurs in a constant volume system.
It has been postulated that the rate expression is of the form
where the partial pressures of ammonia and hydro
gen are expressed in consistent units. The effect of the
reverse reaction may be neglected.
a Determine the manner in which these data should be plot
ted so that a straight line will result if the form of the
postulated rate expression is correct. Use an integrated
form of the rate expression in determining the requisite
functional form.
b Work up the data in tabular form and prepare a plot using
the coordinates determined in part a
c Are the data consistent with the rate expression postu
lated?
d If the data are consistent, what is the corresponding value
of the rate constant?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


