Question
3. (35%) Calculate for 1 mole: a) Entropy of SIC at 800C. b) Entropy change of SIC when it is cooled from 800C to
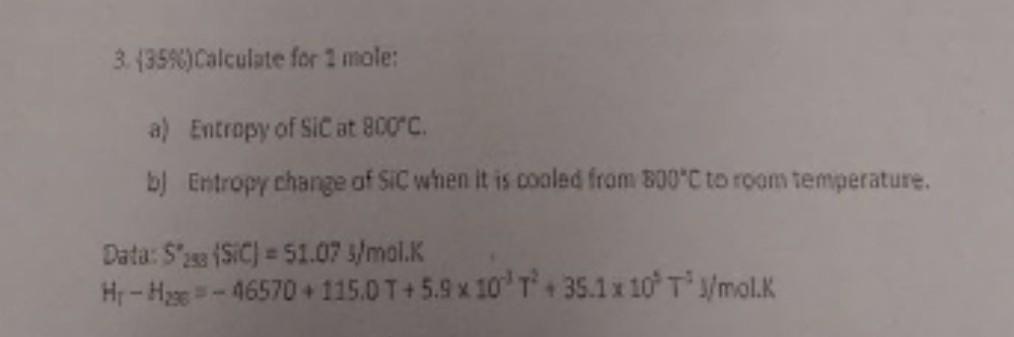
3. (35%) Calculate for 1 mole: a) Entropy of SIC at 800C. b) Entropy change of SIC when it is cooled from 800C to room temperature. Data: S2 (SIC) = 51.07 s/mol.K Hr-Hage-46570+ 115.0 T+5.9 x 10 T+ 35.1 x 10' T'J/mol.K
Step by Step Solution
There are 3 Steps involved in it
Step: 1
1entropy delta HT 465701073 1150 000591073 3511051073 2 T CP 2 dT ds Of dr ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus Early Transcendentals
Authors: James Stewart
8th edition
1285741552, 9781305482463 , 978-1285741550
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



