Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. 4. Consider the species CH4, NH and BH. Choose the correct option with respect to the there species (a) They are isoelectronic and
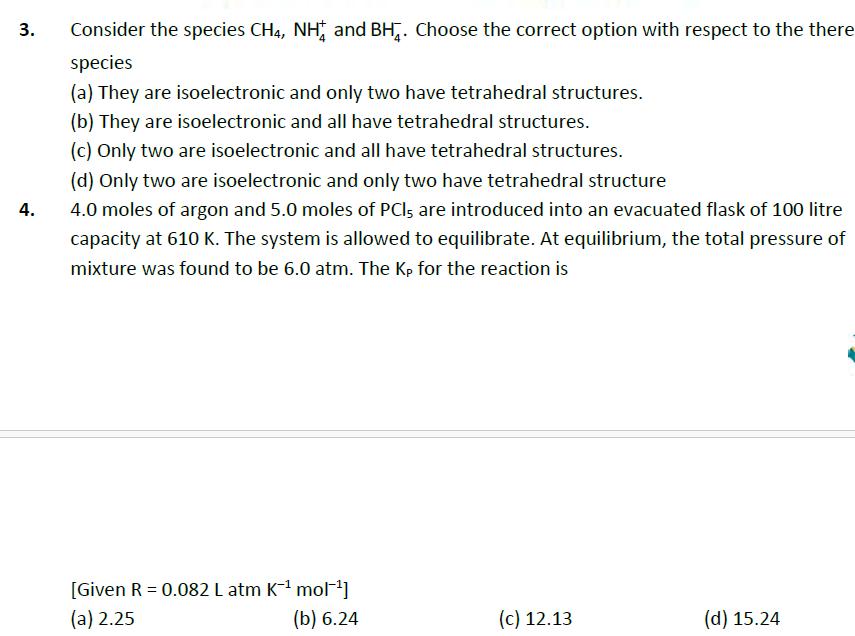
3. 4. Consider the species CH4, NH and BH. Choose the correct option with respect to the there species (a) They are isoelectronic and only two have tetrahedral structures. (b) They are isoelectronic and all have tetrahedral structures. (c) Only two are isoelectronic and all have tetrahedral structures. (d) Only two are isoelectronic and only two have tetrahedral structure 4.0 moles of argon and 5.0 moles of PCI, are introduced into an evacuated flask of 100 litre capacity at 610 K. The system is allowed to equilibrate. At equilibrium, the total pressure of mixture was found to be 6.0 atm. The Kp for the reaction is [Given R = 0.082 L atm K- mol] (a) 2.25 (b) 6.24 (c) 12.13 (d) 15.24
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


