Answered step by step
Verified Expert Solution
Question
1 Approved Answer
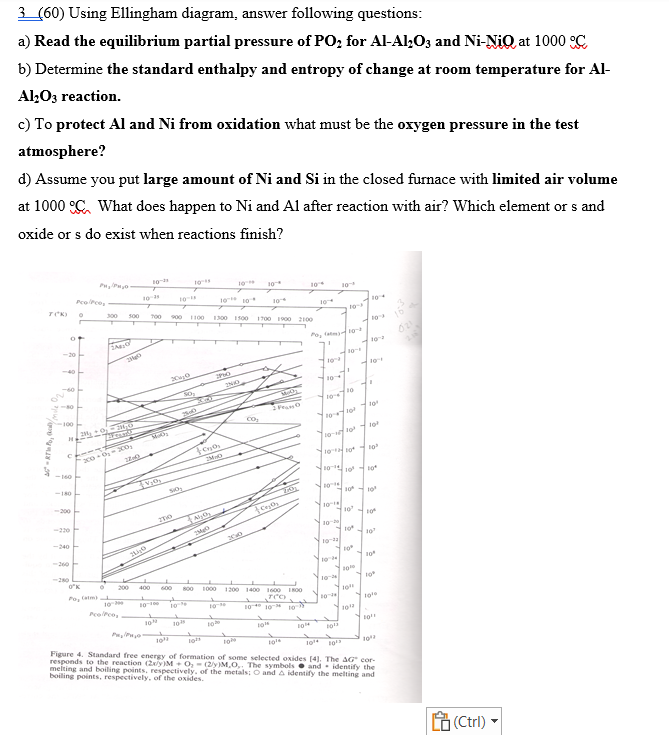
3 ( 6 0 ) Using Ellingham diagram, answer following questions: a ) Read the equilibrium partial pressure of P O 2 for A l
Using Ellingham diagram, answer following questions:
a Read the equilibrium partial pressure of for and NiO at
b Determine the standard enthalpy and entropy of change at room temperature for Al
reaction.
c To protect and from oxidation what must be the oxygen pressure in the test
atmosphere?
d Assume you put large amount of and in the closed furnace with limited air volume
at What does happen to and after reaction with air? Which element or and
oxide or s do exist when reactions finish?
Figure Standard free energy of formation of some selected oxides The cor
responds to the reaction The symbols and identify the melting and boiling points, respectively, of
melting and boiling points, respectively, of the metals: and identify the melting and
boiling points, respectively, of the oxides.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


