Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. A compressor system takes atmospheric air at 5.0 C and supplies it to an air distribution system. The system is at steady state.
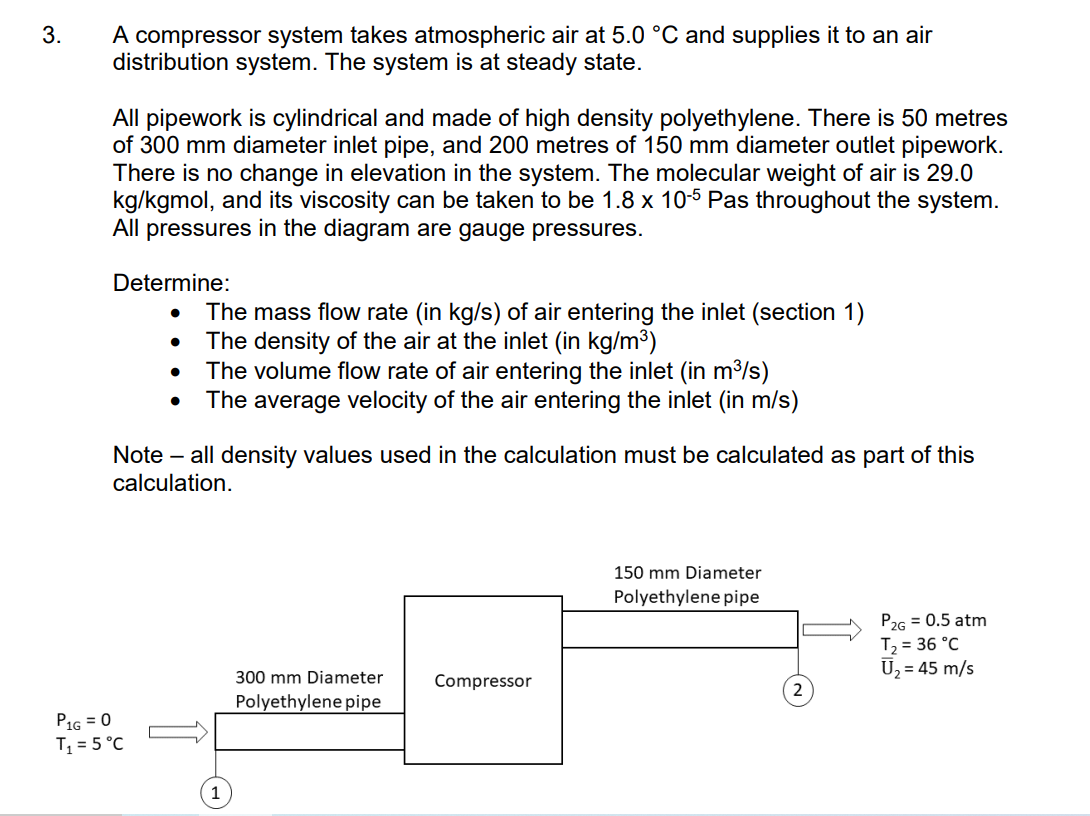
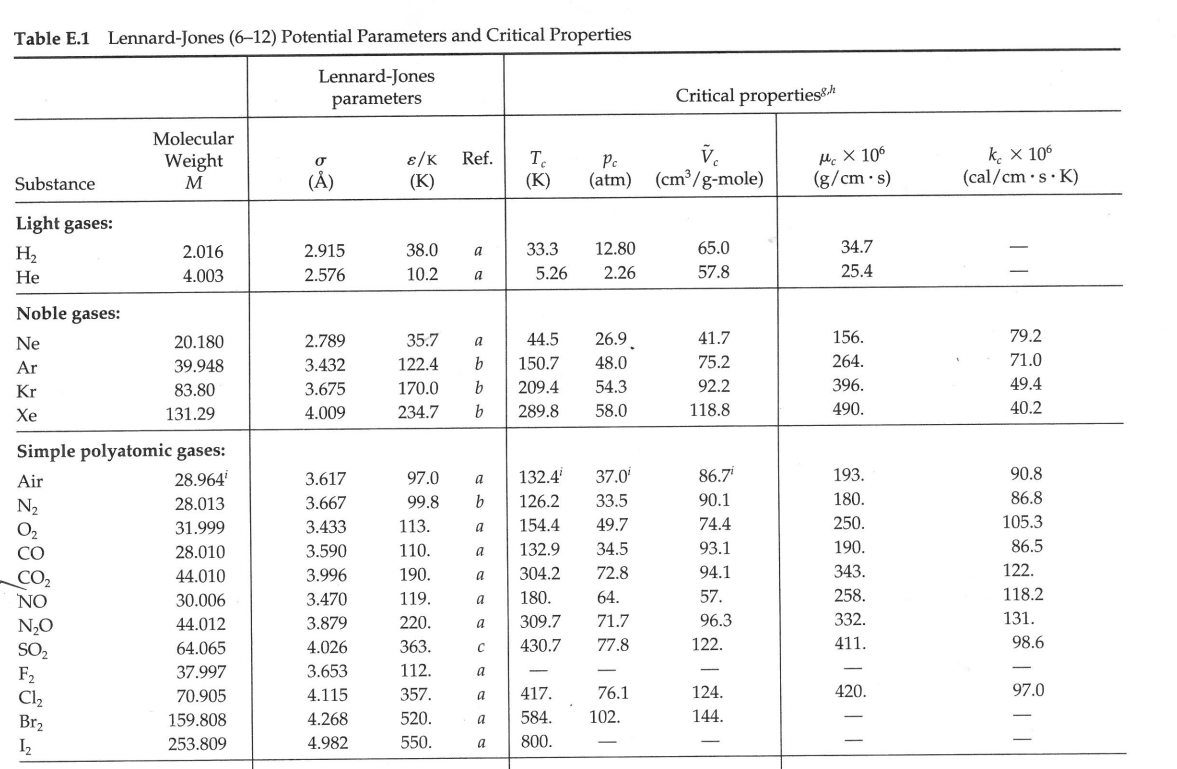

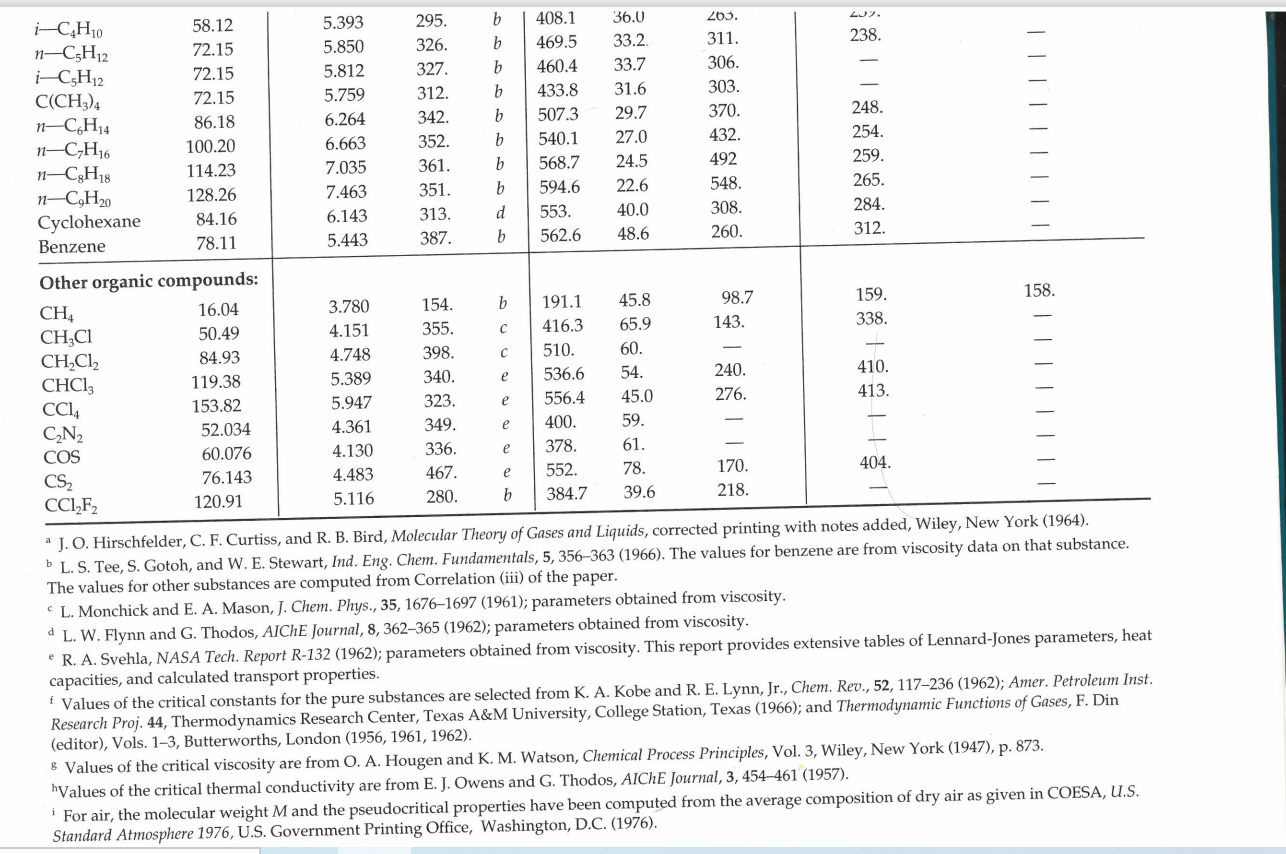
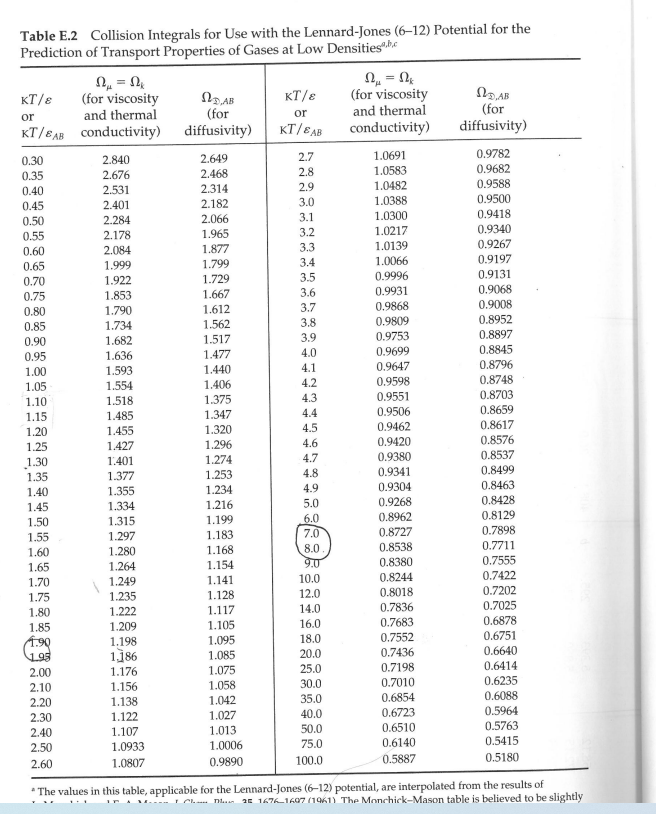
3. A compressor system takes atmospheric air at 5.0 C and supplies it to an air distribution system. The system is at steady state. All pipework is cylindrical and made of high density polyethylene. There is 50 metres of 300 mm diameter inlet pipe, and 200 metres of 150 mm diameter outlet pipework. There is no change in elevation in the system. The molecular weight of air is 29.0 kg/kgmol, and its viscosity can be taken to be 1.8 x 10-5 Pas throughout the system. All pressures in the diagram are gauge pressures. Determine: The mass flow rate (in kg/s) of air entering the inlet (section 1) The density of the air at the inlet (in kg/m) The volume flow rate of air entering the inlet (in m/s) The average velocity of the air entering the inlet (in m/s) Note - all density values used in the calculation must be calculated as part of this calculation. P1G = 0 T = 5C 1 300 mm Diameter Polyethylene pipe Compressor 150 mm Diameter Polyethylene pipe P2G = 0.5 atm T = 36C U = 45 m/s Table E.1 Lennard-Jones (6-12) Potential Parameters and Critical Properties Lennard-Jones Molecular Weight Substance M () parameters Critical properties 87 /K Ref. Pc V (K) (K) (atm) (cm/g-mole) Mc X 106 (g/cms) kc x 106 (cal/cm s.K) Light gases: H 2.016 2.915 38.0 a 33.3 12.80 65.0 34.7 He 4.003 2.576 10.2 a 5.26 2.26 57.8 25.4 | | Noble gases: Ne 20.180 2.789 35.7 Ar 39.948 3.432 122.4 90 a 44.5 26.9 41.7 156. 79.2 b 150.7 48.0 75.2 264. 71.0 Kr 83.80 3.675 170.0 b 209.4 54.3 92.2 396. 49.4 Xe 131.29 4.009 234.7 b 289.8 58.0 118.8 490. 40.2 Simple polyatomic gases: Air 28.964 3.617 97.0 a 132.4' 37.0' 86.7i 193. 90.8 N 28.013 3.667 99.8 b 126.2 33.5 90.1 180. 86.8 02 31.999 3.433 113. a 154.4 49.7 74.4 250. 105.3 CO 28.010 3.590 110. a 132.9 34.5 93.1 190. 86.5 CO2 44.010 3.996 190. a 304.2 72.8 94.1 343. 122. NO 30.006 3.470 119. a 180. 64. 57. 258. 118.2 9 NO 44.012 3.879 220. a 309.7 71.7 96.3 332. 131. SO2 64.065 4.026 363. C 430.7 77.8 122. 411. 98.6 F2 37.997 3.653 112. a - - - - - Cl 70.905 4.115 357. a 417. Br 159.808 4.268 520. a 584. 102. 76.1 124. 144. 420. 97.0 253.809 4.982 550. a 800. - - 158. 203. g| | | | | | Hydrocarbons: CH 16.04 3.780 154. b 191.1 45.8 98.7 159. CH=CH 26.04 4.114 212. d 308.7 61.6 112.9 237. CH2=CH 28.05 4.228 216. b 282.4 50.0 124. 215. CH 30.07 4.388 232. b 305.4 48.2 148. 210. CH,C=CH 40.06 4.742 261. d 394.8 - - - CH,CH=CH 42.08 4.766 275. b 365.0 45.5 181. 233. CH 44.10 4.934 273. b -CH 58.12 5.604 304. 60 369.8 41.9 200. 228. b 425.2 37.5 255. 239. i-CH 58.12 5.393 295. b 408.1 36.0 265. 257. n-CH12 72.15 5.850 326. b 469.5 33.2. 311. 238. i-C,H 72.15 5.812 327. b 460.4 33.7 306. C(CH) 72.15 5.759 312. b 433.8 31.6 303. Chu 86.18 6.264 342. b 507.3 29.7 370. 248. -CH 100.20 6.663 352. b 540.1 27.0 432. 254. -CH 114.23 7.035 361. b 568.7 24.5 492 259. -CH 128.26 7.463 351. b 594.6 22.6 548. 265. Cyclohexane 84.16 6.143 313. d 553. 40.0 308. 284. Benzene 78.11 5.443 387. b 562.6 48.6 260. 312. Other organic compounds: CH4 16.04 3.780 154. b 191.1 45.8 98.7 159. 158. CH,CI 50.49 4.151 355. C 416.3 65.9 143. 338. CHCl 84.93 4.748 398. 510. 60. CHCI, 119.38 5.389 340. e 536.6 54. 240. 410. CCl4 153.82 5.947 323. e 556.4 45.0 276. 413. CN 52.034 4.361 349. e 400. 59. COS 60.076 4.130 336. e 378. 61. CS2 76.143 4.483 467. e 552. 78. 170. 404. CCI-F 120.91 5.116 280. b 384.7 39.6 218. a J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, corrected printing with notes added, Wiley, New York (1964). b L. S. Tee, S. Gotoh, and W. E. Stewart, Ind. Eng. Chem. Fundamentals, 5, 356-363 (1966). The values for benzene are from viscosity data on that substance. The values for other substances are computed from Correlation (iii) of the paper. CL. Monchick and E. A. Mason, J. Chem. Phys., 35, 1676-1697 (1961); parameters obtained from viscosity. d L. W. Flynn and G. Thodos, AIChE Journal, 8, 362-365 (1962); parameters obtained from viscosity. R. A. Svehla, NASA Tech. Report R-132 (1962); parameters obtained from viscosity. This report provides extensive tables of Lennard-Jones parameters, heat capacities, and calculated transport properties. f Values of the critical constants for the pure substances are selected from K. A. Kobe and R. E. Lynn, Jr., Chem. Rev., 52, 117-236 (1962); Amer. Petroleum Inst. Research Proj. 44, Thermodynamics Research Center, Texas A&M University, College Station, Texas (1966); and Thermodynamic Functions of Gases, F. Din (editor), Vols. 1-3, Butterworths, London (1956, 1961, 1962). 8 Values of the critical viscosity are from O. A. Hougen and K. M. Watson, Chemical Process Principles, Vol. 3, Wiley, New York (1947), p. 873. "Values of the critical thermal conductivity are from E. J. Owens and G. Thodos, AIChE Journal, 3, 454-461 (1957). For air, the molecular weight M and the pseudocritical properties have been computed from the average composition of dry air as given in COESA, U.S. Standard Atmosphere 1976, U.S. Government Printing Office, Washington, D.C. (1976). = Nk Table E.2 Collision Integrals for Use with the Lennard-Jones (6-12) Potential for the Prediction of Transport Properties of Gases at Low Densities.c KT/8 (for viscosity KT/e or and thermal KT/BAB conductivity) (for diffusivity) or KT/BAB (for viscosity and thermal conductivity) (for diffusivity) 0.30 2.840 2.649 2.7 1.0691 0.9782 0.35 2.676 2.468 2.8 1.0583 0.9682 0.40 2.531 2.314 2.9 1.0482 0.9588 0.45 2.401 2.182 3.0 1.0388 0.9500 0.50 2.284 2.066 3.1 1.0300 0.9418 0.55 2.178 1.965 3.2 1.0217 0.9340 0.60 2.084 1.877 3.3 1.0139 0.9267 0.65 1.999 1.799 3.4 1.0066 0.9197 0.70 1.922 1.729 3.5 0.9996 0.9131 0.75 1.853 1.667 3.6 0.9931 0.9068 0.80 1.790 1.612 3.7 0.9868 0.9008 0.85 1.734 1.562 3.8 0.9809 0.8952 0.90 1.682 1.517 3.9 0.9753 0.8897 0.95 1.636 1.477 4.0 0.9699 0.8845 1.00 1.593 1.440 4.1 0.9647 0.8796 1.05 1.554 1.406 4.2 0.9598 0.8748 1.10 1.518 1.375 4.3 0.9551 0.8703 1.15 1.485 1.347 4.4 0.9506 0.8659 1.20 1.455 1.320 4.5 0.9462 0.8617 1.25 1.427 1.296 4.6 0.9420 0.8576 1.30 1.401 1.274 4.7 0.9380 0.8537 1.35 1.377 1.253 4.8 0.9341 0.8499 1.40 1.355 1.234 4.9 0.9304 0.8463 1.45 1.334 1.216 5.0 0.9268 0.8428 1.50 1.315 1.199 6.0 0.8962 0.8129 1.55 1.297 1.183 7.0 0.8727 0.7898 1.60 1.280 1.168 8.0. 0.8538 0.7711 1.65 1.264 1.154 9.0 0.8380 0.7555 1.70 1.249 1.141 10.0 0.8244 0.7422 1.75 1.235 1.128 12.0 0.8018 0.7202 1.80 1.222 1.117 14.0 0.7836 0.7025 1.85 1.209 1.105 16.0 0.7683 0.6878 1.90 1.198 1.095 18.0 0.7552 0.6751 1.95 1,186 1.085 20.0 0.7436 0.6640 2.00 1.176 1.075 25.0 0.7198 0.6414 2.10 1.156 1.058 30.0 0.7010 0.6235 2.20 1.138 1.042 35.0 0.6854 0.6088 2.30 1.122 1.027 40.0 0.6723 0.5964 2.40 1.107 1.013 50.0 0.6510 0.5763 2.50 1.0933 1.0006 75.0 0.6140 0.5415 2.60 1.0807 0.9890 100.0 0.5887 0.5180 *The values in this table, applicable for the Lennard-Jones (6-12) potential, are interpolated from the results of 25 1676-1607 (1961) The Monchick-Mason table is believed to be slightly Ch
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solutions Step 1 The concept of conservation of mass which stipulates that the mass flow rate of a fluid entering a system must equal the mass flow ra...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


