Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. (a) Draw the best Lewis diagram for the molecule with the condensed formula CH3C(O)OCH3. (b) There is a resonance structure for CH3C(O)COH3 for

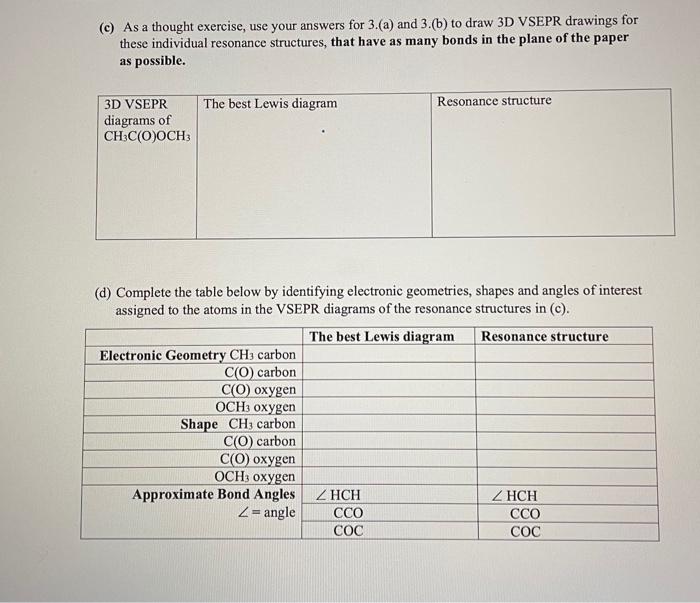
3. (a) Draw the best Lewis diagram for the molecule with the condensed formula CH3C(O)OCH3. (b) There is a resonance structure for CH3C(O)COH3 for which all atoms other than H possess an octet of electrons. Draw this resonance structure. (c) As a thought exercise, use your answers for 3.(a) and 3.(b) to draw 3D VSEPR drawings for these individual resonance structures, that have as many bonds in the plane of the paper as possible. 3D VSEPR The best Lewis diagram diagrams of CH3C(O)OCH3 Resonance structure (d) Complete the table below by identifying electronic geometries, shapes and angles of interest assigned to the atoms in the VSEPR diagrams of the resonance structures in (c). The best Lewis diagram Electronic Geometry CH3 carbon C(O) carbon C(O) oxygen OCH3 oxygen Shape CH3 carbon. C(O) carbon Resonance structure C(O) oxygen OCH3 oxygen Approximate Bond Angles ZHCH ZHCH Z= angle CCO CCO COC COC
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


